More Information
Submitted: October 06, 2024 | Approved: February 08, 2024 | Published: February 09, 2024
How to cite this article: de Oliveira Santos LTS, Sampaio KF, Esposito E, Paulo EM, Góes-Neto A, et al. Isolation and Influence of Carbon Source on the Production of Extracellular Polymeric Substance by Bacteria for the Bioremediation of Heavy Metals in Santo Amaro City. Ann Civil Environ Eng. 2024; 8: 012-017.
DOI: 10.29328/journal.acee.1001060
Copyright License: © 2024 de Oliveira Santos LTS, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Bioremediation; Heavy metals; Exopolysaccharide; Adsorption; Bacteria; Subaé river
Abbreviations: EPS: Exopolissacarídeo; pH: Potencial Hidrogeniônico; FAAS: Flame Atomic Absorption Spectrometry
Isolation and Influence of Carbon Source on the Production of Extracellular Polymeric Substance by Bacteria for the Bioremediation of Heavy Metals in Santo Amaro City
Leila Thaise Santana de Oliveira Santos1*, Kayque Frota Sampaio2, Elisa Esposito3, Elinalva Maciel Paulo1, Aristóteles Góes-Neto4, Amanda da Silva Souza1 and Taise Bomfim de Jesus1
1Biological Sciences Department, State University of Feira de Santana, Bahia, Brazil
2Department of Technology, State University of Feira de Santana, Bahia, Brazil
3Department of Microbiology, Federal University of São Paulo, São Paulo, Brazil
4Department of Microbiology, Federal University of Minas Gerais, Minas Gerais, Brazil
*Address for Correspondence: Leila Thaise Santana de Oliveira Santos, Biological Sciences Department, State University of Feira de Santana, Bahia, Brazil, Email: [email protected]
The city of Santo Amaro (Bahia, Brazil) gained visibility among the scientific community due to the contamination of the Subaé River by lead and cadmium from the PLUMBUM Mineração e Metalurgia Ltda industry, on the banks of the river in 1956, which produced lead ingots The present work aimed to investigate the adsorption capacity of heavy metals (Pb and Cd) of EPS produced by bacterial species from the Subaé River, for possible future application of these biopolymers in bioremediation processes in areas impacted by the aforementioned heavy metals. Subaé river water was collected for physical-chemical analysis and bacterial isolation. It was verified that all isolated bacteria produced an expressive amount of Exopolysaccharide (EPS). Thus, the optimization of this production in different sugars (sucrose, glucose, and mannitol) and in three different pHs: 5.5; 6.5, and 7.5. All bacteria produced EPS in large quantities and the best sugar was sucrose at pH 7.5. In order to use the EPS for the bioremediation area, the adsorption test of lead and cadmium was carried out by the isolated EPS. 0.5 g of the EPS was dissolved in 50 ml of deionized water, then the solutions of metals, lead acetate, and cadmium sulfate (procedure performed separately) were incubated at 28 °C for 16 h after that period, and were centrifuged. Samples were filtered to separate the insoluble EPS and the filtrates obtained were used in the quantification of the metals by atomic absorption (FAAS- Flame Atomic Absorption Spectrometry). Bacillus spp., Bacillus cereus, Staphylococcus spp., and Serratiamarcescens, all showed tolerance to the tested metals, due to the efficiency in the adsorption capacity of the EPS, and it was possible to distinguish seven genera, Klebsiella pneumonia, Pseudomonas aeruginosa, Lysinibacillus spp. to be used in the bioremediation of environments contaminated with heavy metals.
The contamination of natural waters by heavy metals has been one of the major problems of society due to the increase in industrial activities and great efforts are being devoted to the development of ecologically more appropriate, effective, and low-cost technologies for the remediation of contaminated environments. The Subaé-BA river, whose sources are in Feira de Santana and mouth in Salvador, had its waters contaminated by heavy metals with the installation of PLUMBUM Mineração e Metalurgia LTDA in 1956, on the banks of the Subaé River and 10 km from its mouth at Todos os Santos Bay (BTS) [1].
During the production process of lead ingots at the factory, the slag generated was composed of heavy metals, such as arsenic, cadmium, bismuth, and, mainly, lead, which were deposited in the environment where they were subject to chemical or biological weather. The activities of this company lasted until 1993, the year of its closure.
Heavy metals have received special attention due to toxicity and the consequent accumulation in the ecosystem throughout the food chain, thus making the search for new technologies and techniques more efficient and economical that allow the removal of these chemical elements from the contaminated environment. Bioremediation emerges in this context as a viable alternative for the recovery of areas contaminated by heavy metals, since it is gaining more and more importance, due to the advantages it offers such as simplicity, efficiency, and low cost. The bioremediation of degraded ecosystems is the first step in research aimed at using microbial activity to convert toxic substances into less harmful compounds [2].
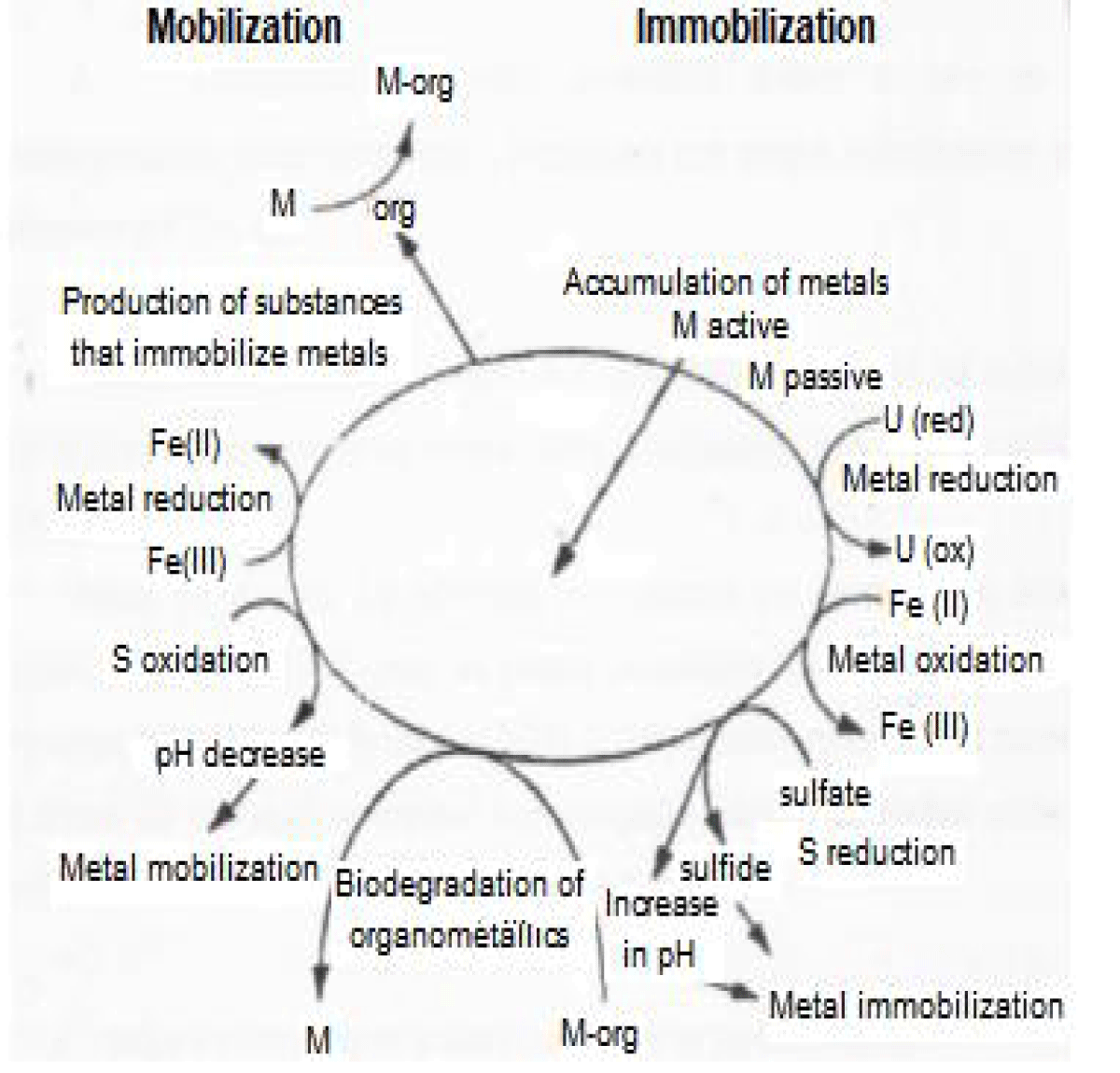
In this context, some bacteria are promising candidates for having specialized systems for resistance to metals. Among the mechanisms of resistance to metals, we have bioaccumulation, biosorption, reduction, production of siderophores, and formation of biofilms and biofilters (Figure 1), which can be explored for the development of clean technologies for the control of metal pollution in order to promote mitigation of environmental impacts [3].
Biofilms, for example, can be defined as a set of microorganisms involved in a matrix that consists of a mixture of polymeric compounds, mainly polysaccharides known as Exopolysaccharides (EPS) [4]. EPS has the function of protecting the bacterial cell against desiccation and phage attack, as well as antibiotics, toxic and protozoan compounds, sequester essential cations, and involvement in the adhesion on solid surfaces, in addition to the formation of biofilms. Another possible function of EPS is that it can be excreted in response to environmental stressors, such as exposure to heavy metals, reducing its toxicity. They are also capable of acting on the colonization of surfaces and, therefore, they can have a surfactant action, which allows their use as surfactants or emulsifiers and confer potential for industrial use and in bioremediation processes [5,6].
This research was conducted to assess the impact of heavy metals in Rio, to isolate bacteria tolerant to heavy metals, and with a mechanism that could be applied to the decontamination of water impacted by heavy metals. Only 8 bacterial isolates were obtained, but all with Pb and Cd adsorption capacity in EPS. From these data, we sought to evaluate the resistance mechanisms of microorganisms and techniques in the bioremediation of surface waters contaminated with lead (Pb) and cadmium (Cd).
EPS production
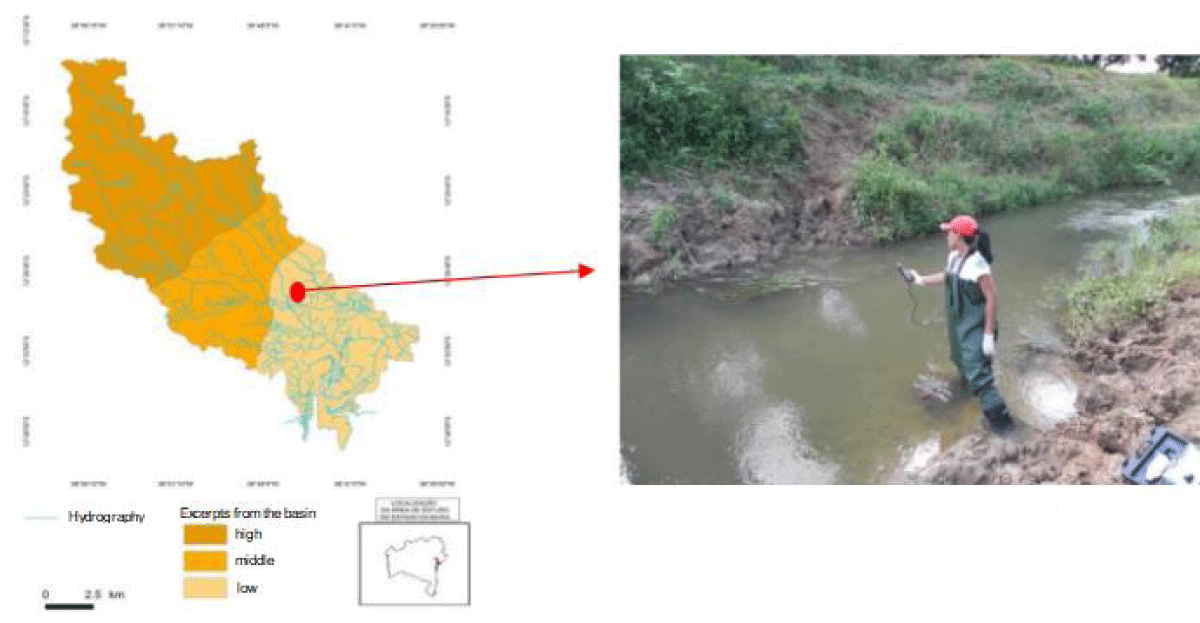
In May 2013, surface water samples from the Subaé River (Figure 1), in the Santo Amaro region, were collected approximately 500 m from the PLUMBUM factory, to determine the physical-chemical and microbiological characteristics. The collection followed the standards described in the Standard Methods for Water and Wastewater Examination [7]. 900 mL of surface water samples were collected at 6.1 m from the riverbank, in sterile glass bottles of 1 L capacity. From the water samples, eight bacteria were isolated and maintained in the laboratory, Klebsiella pneumoniae CCMB716, Pseudomonas aeruginosa CCMB717, Lysinibacillus spp. CCMB718, Bacillus spp. CCMB719, Bacillus cereus CCMB720, Staphylococcus spp. CCMB721 and Serratia marcescens MBR7 and tested for the production of EPS in three sugars (Figure 2).
Figure 1: Interaction between metals and microorganisms. Source: Ledin (2000)
Figure 2: Map of the location of water collections in the lower course of the Hydrographic Basin of the Subaé River in the region of Santo Amaro-BA.
EPS production was carried out in the base medium optimized by Guimarães, et al. [8], with some adaptations, with sugars varying from 10% (sucrose, glucose, and mannitol), pH (5.5, 6.5, and 7.5) and the standard incubation temperature of 28 °C. The readings were performed with 24 hours of incubation. The method used was the one developed by Paulo [9]. This technique consists of inoculating 5μL of the active culture of each isolate [suspended in saline at an absorbance of 0.96 (+/- 600 nm)] in sterile filter paper discs (5 mm Ø), deposited on the solid culture medium. After the incubation period, a reading was made based on the formation of dense colonies on the discs.
The level of biopolymer production was evaluated according to the diameter of the colony: from 0 to 6.9 mm considered without biopolymer production; from 7 mm to 14.9 mm, little production and above 15 mm, as great production. A rapid test was carried out to confirm the production of EPS, mixing a range of the potentially biopolymer-producing colony in 2 mL of absolute ethanol. Half the ethanol capacity was placed in a glass test tube and with the platinum handle it took the contents around the disc (the viscous material that formed on the discs inserted in the EPS medium), and transferred it to the tube with ethanol so that this content comes off the platinum handle.
The formation of a precipitate in the medium was indicative of the production of polysaccharide-type biopolymers. So, if there was precipitation without turbidity, it is because the content that grew at the bottom of the tube is EPS. If there was only turbidity, it is because the contents of the disc are bacterial colony mass.
When testing the production of EPS with absolute ethanol, the result was positive for all eight bacteria isolated. Thus, after confirmation, isolates were selected that had the highest amount of mucoid substance for testing under different conditions. For this analysis, the pHs (5.5, 6.5, and 7.5), incubation temperature (28 ºC, 35 ºC and 42 °C), and the carbon sources (sucrose, glucose, and mannitol) at a concentration of 10% were varied, in order to verify which of these conditions would favor greater EPS production.
The culture medium used was the same used for the initial selection of EPS production, with variations of the conditions mentioned. For each isolate, the experiment was carried out in triplicate. The experimental analysis consisted of measuring, with a caliper, the diameter of the mucoid layer around the filter discs and to optimize the tested conditions, the DOEHLERT model was used [10].
Biochemical characterization of EPS
The biochemical characterization of EPS was performed, its biochemical groups were analyzed and its potential for adsorption of heavy metals was tested since the biosorption process occurs as a result of the interactions of metals and/or organic pollutants with the functional groups present in the constituent organic polymers cell surface or as a result of metabolic processes.
Dry EPS analysis was performed by Fourier transform Infrared Spectroscopy (FTIV OR FTIR) with attenuated total reflectance (ATR-FTIR) in Shimadzu's IRAffinity-1 FTIR equipment, at Unifesp São José dos Campos, Instituto de Ciência e Environmental Technology / Biotechnology. Based on Ruschel, et al. [11], the EPS spectra were obtained as follows: first 5 mg of the sample was spread over the surface of the ATR crystal, then the spectrum was obtained. A simple and quick instrumental technique that can show different functional groups of a molecule, as it depends on the interaction of the molecule with electromagnetic radiation in the Infrared (IV) region, and the absorption of electromagnetic radiation in the IV region interferes with the vibrations of the connections covalent, increasing their amplitude.
EPS production for metal adsorption (Lead and Cadmium)
The bacteria were activated in Broth Müeller Hinton (CMH) medium at 37 °C for 24 h. After that period, the bacteria were suspended in 0.85% saline until an absorbance of 0.96 (+/- 600 nm) was obtained. Then, 1% of the bacterial solution was inoculated in 100 mL of EPS medium, pH 7.0, and incubated at 28 °C for 24 h, with and without shaking at 150 rpm. All tests were done in triplicate. The extraction of the EPS produced occurred in 02 ways: one with the use of 10% trichloroacetic acid (TCA), CCl3COOH, and the other without TCA. TCA is widely used in biochemistry for the precipitation of macromolecules such as proteins, DNA, and RNA. Since EPS without TCA contains proteins and with TCA does not, thus, we analyzed whether protein groups interfere or not with metal adsorption, as TCA has a fragmenting effect on the protein chains present in the produced polymers.
For EPS extracted with 10% TCA, 100 ml of 10% TCA was added to each 100 ml of the medium with the inoculated culture, maintaining the ratio (1: 1), stirred in the shaker at 90 rpm, 25 °C +/- 2 for 30 minutes.
EPS production
All bacteria produced EPS in large quantities and the best sugar was sucrose with pH 7.5, a condition in which the EPS production diameter exceeded 18 mm (Table 1).
| Table 1: EPS production in different carbohydrates and pH of bacterial isolates: Klebsiella pneumoniae, Pseudomonas aeruginosa, Lysinibacillus spp., Bacillus spp., Bacillus cereus, Staphylococcus spp. and Serratia marcescens from samples collected from the banks of the Subaé river in the municipality of Santo Amaro / BA. | ||
| Carbon Source | pH | Isolates producing the EPS/Ø |
| Glucose | 5.5 6.5 7.5 |
All (above 15 mm) All (above 15 mm)) None produced |
| Sucrose | 5.5 6.5 7.5 |
All (above 15 mm) All (above 15 mm) All (above 15 mm) |
| Mannitol | 5.5 6.5 7.5 |
All (above 15 mm) All (above 15 mm) All (above 15 mm) |
Among the tested carbon sources, sucrose provided a more expressive production of EPS. This result corroborates with data found in the literature, where it is observed that glucose and sucrose are the most used carbon sources for the production of EPS [12-17]. However, there is a cultivation time and an optimal concentration of carbon source for the production of EPS for each microorganism [18]. The pH variation did not differ, with pH 7.5 being defined for EPS production.
Of all the bacteria, Pseudomonas aeruginosa was considered the best isolate for the production of EPS (Sucrose 10% at 28 ºC) with TCA 10%, as it presented greater dry weight under the conditions tested. For this reason, the P. aeruginosa bacterium was selected to produce EPS to perform the metal retention test to elaborate the Biofilter. The yields obtained are compatible with other findings in the literature, where the constant dry weight varies from 0.2512 to 2, 2130 g EPS [19] obtained 2.2000 g in the production of EPS [20], found 1.5750 g in strains of Pseudomonas ssp., Demonstrating that the methodology of the experiments could verify the interaction of the parameters to obtain a better response, which minimizes analysis time and improves conditions for a larger experiment.
Biochemical characterization of EPS
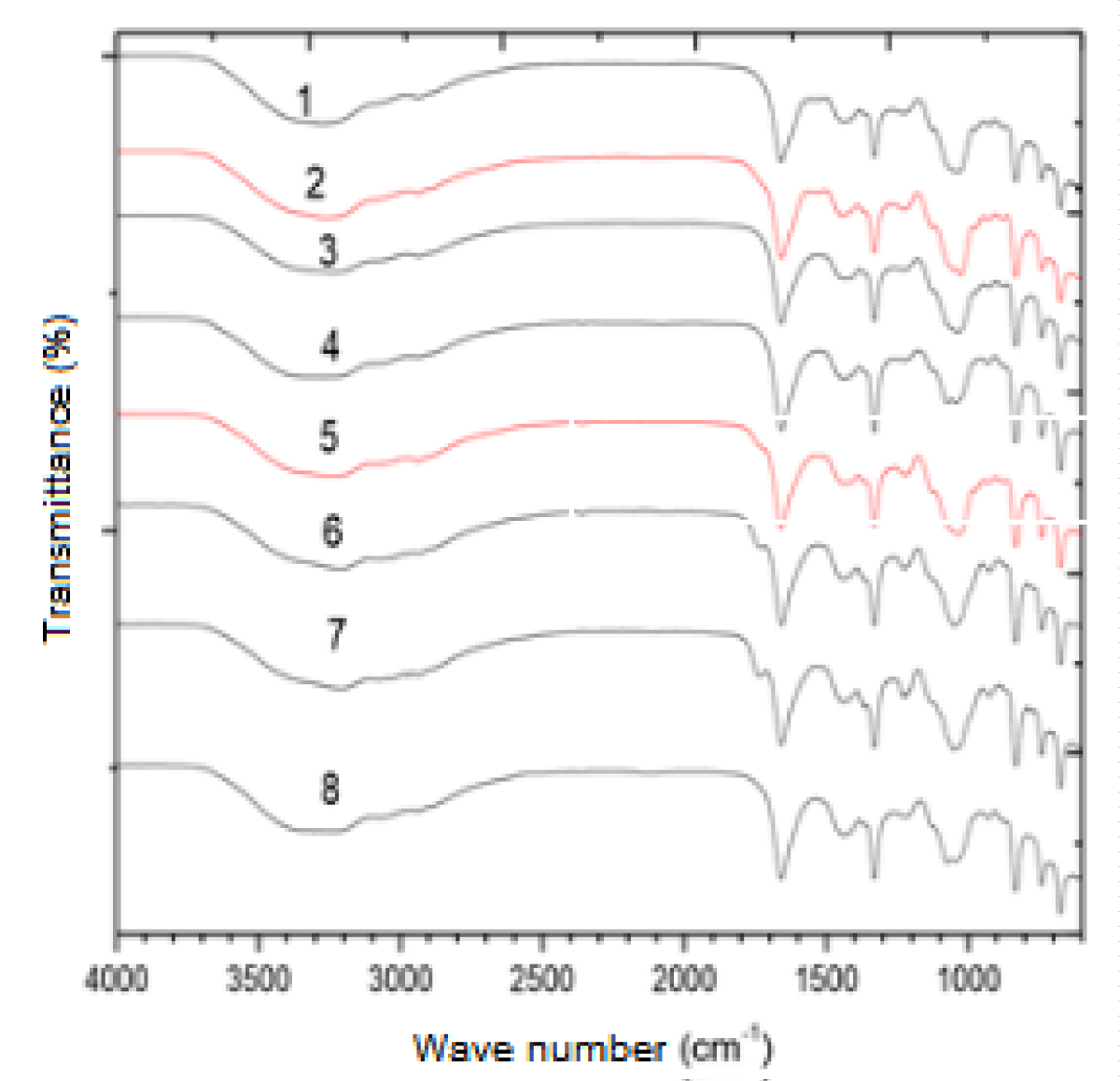
The biochemical characterization of EPS made it possible to analyze some chemical groups that may be interacting with the heavy metals present in the waters from which these bacteria were isolated, facilitating the understanding of their resistance mechanisms in these environments considered extremophiles due to the high degree of environmental contamination. The EPS samples produced by the bacteria showed similar spectra (Figure 3). Correlation tables in IV were used to extract structural information from the spectrum. All have peaks between 3600 cm-1 and 3200 cm-1 corresponding to bands of hydroxyl groups (OH). The second peak is located around 1660 cm-1, indicating that there is a stretch of C = O and C ‒ N (amines and amides in amino acids), which indicates the presence of proteins in the sample. The peak at about 1060 cm-1 again indicates the presence of stretching of the OH group.
Figure 3: Set of EPS infrared spectra of all bactéria.
The carbonyl elongation of carboxylic acids appears close to 1750 cm-1. A band at 1420 cm-1 may be due to the C-OH deformation vibration with the contribution of the O-C-O symmetrical vibrational stretching of the carboxylate group. The bands 1260 cm-1 and 1160 cm-1 can be attributed to the presence of sulfate ester groups (S = O). The peak is around 830 cm-1, present in the samples of the bacteria Bacillus spp., Bacillus cereus, and Staphylococcus spp. indica the presence of a fold in a substituted trialcene.
There are no studies on the characterization of EPS produced by these microorganisms aimed at environmental contamination areas, and the process of biofilm formation, both P. aeruginosa by Staphylococcus aureus or by Serratia marcescens, is further investigated, focused on their impact on the development of resistance to antimicrobials, evidencing the current pioneering research in the production of EPS aimed at bioremediation of environments with heavy metals with these microorganisms.
EPS production for metal adsorption (Lead and Cadmium)
The EPS produced without the addition of 10% TCA by all the tested bacteria was more firm and compacted in the bottom of the containers (showing crystalline visual aspects). One of the vials with the centrifuged content of the colony K. pneumoniae, two of the colony B. cereus, and two of the vials of the colony Serratia marcescens (without the addition of 10% TCA) showed a biphasic visual aspect and during the addition of ethanol (92, 8 °GL) were softer, more flexible and bulky. For Bacillus cereus bacteria, prior to centrifugation, the Erlenmeyer contents were viscous (with a honey consistency of around 59.60 mPa.s) and with EPS precipitates at the bottom of the containers. And for the Serratia marcescens bacteria, before the centrifugation, the colony Staphylococcus spp.
For all eight bacteria, the EPS produced with the addition of 10% TCA was presented visually in the form of a semi-granulated solid material, and their mechanical separation with the liquid phase became difficult, requiring centrifugation to provide this separation. This probably occurred due to the proteolysis that EPS underwent, precipitating low molecular weight peptides and free amino acids, due to the action of TCA 10%, which was enhanced with the use of chilled ethanol.
Adsorption of Pb by EPS produced by microorganisms
In all EPS of the tested bacteria, it was found that the removal efficiency was greater for Cd than for Pb, with adsorption of both Cd and Pb being more efficient by bacterial EPS without TCA (Table 2).
The best adsorption performance of metals was from EPS without TCA extracted from Klebsiella pneumoniae, the maximum biosorption capacity for Cd and Pb was 0.32666 mg L-1 and 0.10339 mg L-1, respectively. This good performance was also verified in the work of [21] whose Pb adsorption was 99.5 mg g-1 by the EPS produced by the genus Klebsiella sp.
EPS TCA was produced with Bacillus spp., Bacillus cereus, and Lysinibacillus spp. did not adsorb Cd. The lowest efficiency for Cd removal was from EPS TCA without Bacillus spp., 0.00001 mg L-1, and the lowest efficiency for adsorbing Pb was from EPS with TCA produced by Pseudomonas aeruginosa and Staphylococcus spp., 0.00012 mg L-1. This fact can be justified by a greater metallic affinity to EPS than to the cell surface in vivo systems since EPS contain ionizable functional groups such as carboxyl, phosphate, amine, and hydroxyl, based on that described in IV, which enable them to kidnap metal ions.
Metals such as Cu+2, Cd+2, Ni+2, Pb+2, Zn+2, Co+2, and Cr+2, due to their similar chemical coordination characteristics, end up competing substantially with each other for biosorbent binding sites [22]. The formation of the weakest links occurs as the strongest sites become saturated. Most studies carried out emphasize the formation of chelating rings, but this mechanism cannot be considered alone, the characterization of EPS in relation to the chemical groups responsible for the adsorption of metals, corroborates in a better understanding of the Bacterial-metal EPS interaction process [23]. However, in this experiment, there was no competitive adsorption of Pb and Cd as the tests were performed separately for each metal. Possibly, the adsorptive behavior of Cd in relation to Pb may be due to its greater metallic affinity and attraction of electrostatic charges to the chemical groups present in EPS, which are influenced by the reactions of oxide-reduction and kinetics of the reactions.
The results demonstrate the efficiency of bacterial EPS isolated in the adsorption of different metallic ionic species from aqueous solutions. In this context, it is worth noting that the existing methods for reducing the concentration of heavy metals in water and effluents present difficulties in application and high cost, in addition to their efficiency being relatively low. In industry, there are few cases in which alternative techniques are used to reduce the levels of these metals in their effluents. In the vast majority of cases, chemical precipitation is the technique used. More efficient processes, such as reverse osmosis, ultrafiltration, and ion exchange, still do not have a relevant practical application in the field of industrial effluent treatment because they are expensive and difficult to operate [24].
Cultivable bacteria identified in the surface waters of the Subaé River in the Santo Amaro-BA stretch, Klebsiella pneumoniae, Pseudomonas aeruginosa, Klebsiella pneumoniae, Lysinibacillus spp., Bacillus sp., Bacillus cereus, Staphylococcus spp., Serratia marcescens have a tolerance to heavy metals and capacity EPS production line.
The EPS of each bacterial strain, tested in isolation, showed high affinity and adsorption capacity, mainly for Cd.
The results showed that the EPS produced by all eight bacteria represents one of the biochemical mechanisms they developed to resist these metals, presenting the potential for bioremediation as a technologically efficient alternative to control the migration of heavy metals along the Subaé River.
In this sense, this work expands the strategies in the control of pollutants and creates tools for the development of new technologies for environmental repairs and recoveries, making it a more detailed study of the characteristics of this material, as well as the variables involved in its behavior during the metal adsorption process.
Highlights
- This work made it possible to discover the bacterial biodiversity of the Subaé River (Santo Amaro-BA)
- Bacteria present in the surface waters of the Subaé River in the Santo Amaro-BA section showed tolerance to heavy metals
- The adsorption capacity of lead and cadmium by the Exopolysaccharide (EPS) produced by all bacterial isolates was verified
- The great potential of bacterial isolates to improve the bioremediation of Pb and Cd in a contaminated system was identified.
We are grateful to FAPESB Process No. 6682/2009 for financing the project and for granting the Ph.D. scholarship in Biotechnology at the State University of Feira de Santana-BA. To the UEFS team and laboratories: LAMASP, LAPEM, LAMOL, LABOTEC, and the Institute of Science and Technology (UNIFESP-SP).
- Andrade MF, Moraes LRS. Lead contamination in santo amaro defies decades of research and delayed reaction on the part of the public authorities. Environment and Society. 2013; 16(2):63-80.
- Souza SL, Carvalho FJPC, Reissmann CB, Gonçalves CG, Krenzynsky M, Staley J. Microbial Diversity and the Biosphere. 1998.
- Stewart PS, Franklin MJ. Physiological heterogeneity in biofilms. Nat Rev Microbiol. 2008 Mar;6(3):199-210. doi: 10.1038/nrmicro1838. PMID: 18264116.
- Abee T, Kovács AT, Kuipers OP, van der Veen S. Biofilm formation and dispersal in Gram-positive bacteria. Curr Opin Biotechnol. 2011 Apr;22(2):172-9. doi: 10.1016/j.copbio.2010.10.016. Epub 2010 Nov 23. PMID: 21109420.
- De Vuyst L, Degeest B. Heteropolysaccharides from lactic acid bacteria. FEMS Microbiol Rev. 1999 Apr;23(2):153-77. doi: 10.1111/j.1574-6976.1999.tb00395.x. PMID: 10234843.
- Looijesteijn PJ, Trapet L, de Vries E, Abee T, Hugenholtz J. Physiological function of exopolysaccharides produced by Lactococcus lactis. Int J Food Microbiol. 2001 Feb 28;64(1-2):71-80. doi: 10.1016/s0168-1605(00)00437-2. PMID: 11252513.
- APHA – AWWA- WPCF. Standard methods for the examination of water and wastewater. 19th edition. Washington D.C. American Public Health Association. 1995; 953.
- Guimarães DP, Costa F, Rodrigues MI., Maugeri F. Optimization of dextran synthesis and acid hydrolysis by surface response analysis. Brazilian Journal of Chemical Engineering. 1999; 16:129-139.
- Paulo EM. Production of exopolysaccharides (EPS) by lactic acid bacteria occurs microencapsulation of Lactobacillus acidophilus La-5 by the Spray-drying process. 2010. 212f. Thesis (Doctorate in Biotechnology) - State University of Feira de Santana, Feira de Santana, 2010.
- Paulo EM, Vasconcelos MP, Oliveira IS, Michelle H, Affe DJ, Nascimento R, Assis SA. An alternative method for screening lactic acid bacteria for the production of exopolysaccharides with rapid confirmation. Food Science and Technology. 2012; 32(4):710–14.
- Ruschel CFC, Huang, CT, Samios D, Ferrão MF. Exploratory analysis applied to attenuated total reflection spectra in Fourier transform infrared (ATR-FTIR) of biodiesel/diesel blends. New Chemistry. 2014; 37(5).
- Seviour RJ, Stasinopoulos SJ, Auer DPF, Gibbs PA. Production of pullulan and other exopolysaccharides by filamentous fungi. Critical Reviews. Biotechnology, Boca Raton. 1992; 12:279-98.
- Schuster R, Wenzig E, Mersmann A. Production of the fungal exopolysaccharide pullulan by batch-wise and continuous fermentation. Applied and Microbiology and Biotechnology. 1993; 39: 155-8.
- Fariña JI, Siñeriz F, Molina OE, Perotti NI. High scleroglucan production by Sclerotiumrolfsii: Influence of medium composition. Biotechnology Letters, Dordrecht. 1998; 20(9):825-31.
- Bae JT, Sinha J, Park JP, Yun JW. Optimization of submerged culture conditions for exo-biopolymer production by Paecilomyces japonica. Journal of Microbiology and Biotechnology, Kangnam-Ku. 2000; 10:482-7.
- Dekker RF, Barbosa AM. The effects of aeration and veratryl alcohol on the production of two laccases by the ascomycete Botryosphaeria sp. Enzyme Microb Technol. 2001 Jan 2;28(1):81-88. doi: 10.1016/s0141-0229(00)00274-x. PMID: 11118601.
- Pigatto MM. Exopolysaccharide production by the ligninolytic fungus Botryosphaeria sp. Monograph - State University of Londrina, Londrina. 2002; 53.
- Barbosa AM, Cunha PDT, Pigatto MM, Silva MLC. Production and applications of fungal exopolysaccharides. Semina: Exact and Technological Sciences, Londrina. 2004; 25(1):29-42.
- Guimarães LB, Santos VZR, Covizzi LG, Garcia-Cruz CH. Influence of different nitrogen sources on growth and EPS production by Diaporthe phaseolorum var. caulivora. Brazilian Journal Food Technology, VII BMCFB, June 2009.
- Miled K, Sab K, Le Roy R. Particle size effect on EPS lightweight concrete compressive strength: experimental investigation and modeling, Mechanics of Materials. 2007; 39.
- Wei W, Wang Q, Li A, Yang J, Ma F, Pi S. Biosorption of Pb (II) from aqueous solution by extracellular polymeric substances extracted from Klebsiella sp. J1: Adsorption behavior and mechanism assessment. Scientific Reports. 2016; 6(31575):1-10.
- Volesky B. Biosorption process simulation tools. Hydrometallurgy. 2003; 71:179-190.
- Girardi F, Hackbarth FV, Souza SMAGU, Souza AAU, Boaventura RAR, Vilar VJP. Marine macroalgae Pelvetia canaliculata (Linnaeus) as natural cation exchanger for metal ions separation: A case study on copper and zinc ions removal. Chem Eng J. 2014; 247:320-29.
- Hackbarth FV, Girardi F, Souza SMAGU, Souza AAU, Boaventura RAR, Vilar VJP. Marine macroalgae Pelvetia canaliculata (Phaeophyceae) as a natural cation exchanger for cadmium and lead ions separation in aqueous solutions. Chem Eng J. 2014; 242:294- 305.