More Information
Submitted: July 01, 2022 | Approved: July 20, 2022 | Published: July 21, 2022
How to cite this article: Kazungu ML, Wekesa NA, Balozi KB, Manyala OJ, Maghanga KJ, et al. Defluoridation of water by the Homa* method, a co-precipitation technique using wood ash leachate and alum. Ann Civil Environ Eng. 2022; 6: 031-039.
DOI: 10.29328/journal.acee.1001037
Copyright License: © 2022 Kazungu ML, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Alum; Defluoridation; Co-precipitation; Homa method; Leachate; Water; Wood ash
Defluoridation of water by the Homa* method, a co-precipitation technique using wood ash leachate and alum
Kazungu ML1, Wekesa NA2, Balozi KB3, Manyala OJ4, Maghanga KJ5 and Etiégni L6*
1Department of Forestry & Wood Science, University of Eldoret, Kenya
2School of Agricultural Sciences & Natural Resources, University of Kabianga, Kericho, Kenya
3Department of Forestry and Wood Science, University of Eldoret, Eldoret, Kenya
4Jaramogi Oginga Odinga University of Science and Technology (JOOUST), School of Spatial Planning and Natural Resource Management, Bondo, Kenya
5Taita-Taveta University, Kenya
6Department of Forestry & Wood Science. University of Eldoret, Eldoret, Kenya
*Homa is the name given to a set of hills located in Western Kenya with hot springs containing high fluoride. This new technique was first applied to water samples from those springs
*Address for Correspondence: Etiégni L, Department of Forestry & Wood Science. University of Eldoret, P. O. Box 1125, Eldoret, Kenya, Email: [email protected]
High fluoride level in drinking water is an endemic public health concern in East Africa. Unlike in Kenya where it is absent, the Nalgonda technique, a defluoridation method that uses two chemicals, alum, and CaO, has seen mixed results in its application and adoption in Ethiopia and Tanzania. This has been due to the low capacity of communities to manage the process and the breakdown in the supply chain of chemicals used in the technique. In the present study, we attempted to bridge the gap in the chemical deficit by investigating the possible substitution of CaO with leachate from wood ash, a by-product of wood combustion commonly found in Kenya. The leachate was prepared from one part of wood ash mixed with two parts of distilled water and stirred for 24 hours followed by decantation. The new technique, the Homa method, using alum and wood ash leachate was then tested on H2O samples from three areas in Kenya with high F- concentrations ranging from 5.1 mg L-1, 9.1 mg L-1 to 91.0 mg L-1. The determination of F- concentration by SPADNS Spectrophotometry was applied throughout the experiment. Four levels of alum i.e. 1%, 2%, 3%, and 4% were dosed on five volumes of water i.e. 100, 200, 300, 400, and 500 ml raw water at 5.1 and 9.1 mg L-1 F-. For water samples at 91.0 mg L-1 F-, the same volumes were treated with 5 higher alum levels i.e. 5%, 6%, 7%, 8%, and 9%. The final pH was then adjusted to 7 with ash leachate for defluoridation. The set-up was a factorial design experiment where the final F- concentration was the dependent variable and the volume of raw water, the percentages, and volume of alum and wood ash leachate constituted the different factors. A fitted multivariate regression model of the general form; where Y = Residual fluoride, X = wood Leachate volume, W = alum Concentration, X*W = Interaction α, β, γ were regression coefficients, ε = error term, showed that only in the Baringo area did we have an interaction between wood ash leachate and alum concentration significant (p < 0.05). Defluoridation occurred (p < 0.05) at as low as 10% and as high as 99%, depending on the initial F- content. Total coliform decreased from 310, 290 and 270 count/l respectively to zero. Unfortunately, high chemical and TDS (from 558 mg L-1 to more than 9,000 mg L-1) enrichment were recorded in addition to the mixed data on turbidity. The overall results show that wood ash can substitute CaO in the Nalgonda process. Further investigation is however required to make it applicable for potable water production.
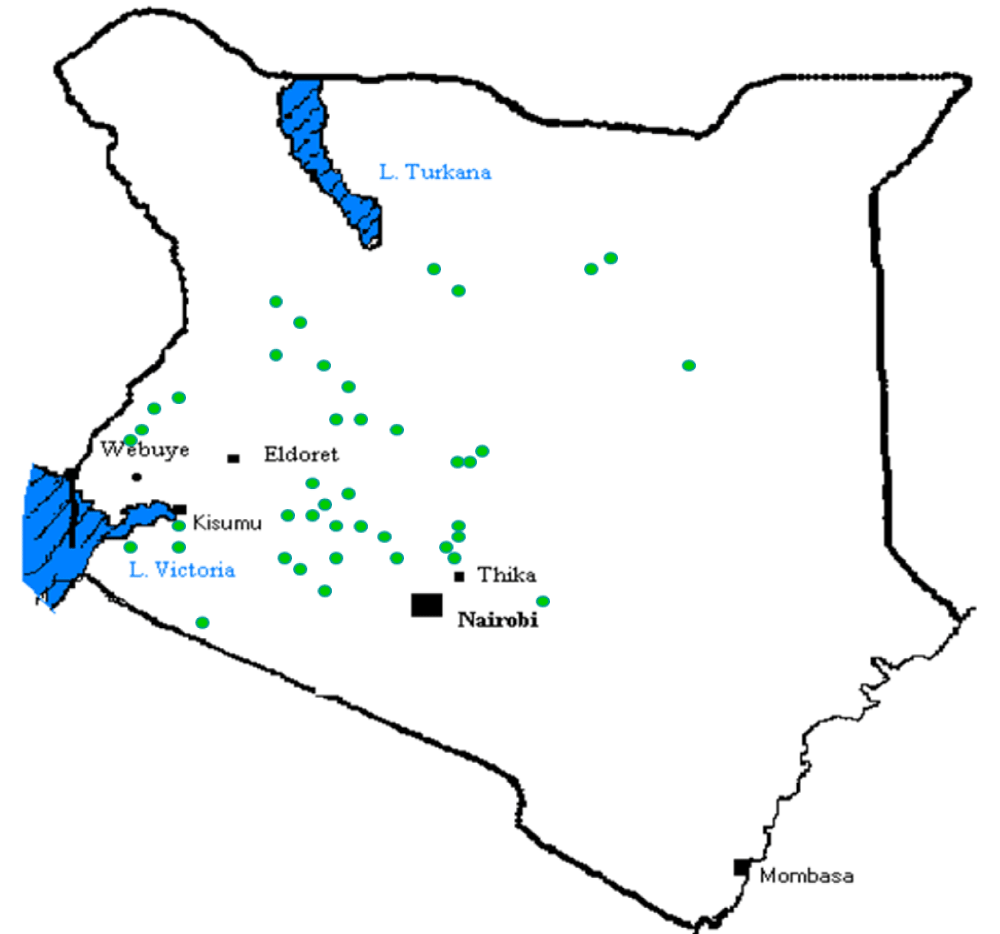
The chemical nature of water is one of the most important parameters, which determine its usefulness and applicability for a specific need. As such not all the waters are fit for domestic use. Many water sources, especially surface and groundwater, in Kenya contain harmful substances in varying concentrations that make the water unsafe and unfit for domestic consumption. For example, the geology of Kenya designates it as one of the countries in the world where fluoride occurs naturally in high concentrations, not only in rocks and soil but also in surface and groundwaters as shown in Figure 1 [1,2]. Higher water fluoride concentrations can also be found in certain springs, boreholes, and a few lakes in the Rift Valley [3,4].
Figure 1: Map of Kenya showing areas with high fluoride concentration in water.
The rapid growth of Kenya’s population, which currently stands at 56 million (71% of which live in rural areas), and changes in rainfall patterns have exacerbated water scarcity and inaccessibility, forcing communities to turn to poor-quality water sources for their needs. It is therefore increasingly difficult to find sufficient fresh water supplies that are fit for human consumption. Access to quality drinking water in many parts of Kenya is consequently limited and, if available, the water contains relatively high fluorides [5,6]. It is estimated that 19.5% of groundwater in Kenya contains fluoride concentrations above 5 mg L-1 [7].
Several epidemiological studies have shown that fluoride in drinking water has a narrow intake range between beneficial and detrimental health effects. Fluoride ingestion in humans is necessary if it does not exceed the 1.5 mg L-1 limit. Excess fluoride consumption causes different types of ailments such as primarily dental and skeletal fluorosis which have been documented in many parts of Western Kenya, Rift Valley, Nyanza, Central and Lower Eastern Provinces [7-9].
Excessive consumption of fluoride can also lead to manifestations of gastrointestinal, neurological, and urinary problems [10-12]. Cases of esophageal cancer, predominantly esophageal squamous cell carcinoma (ESCC), the third most common cause of cancer death in East Africa, have also been partially linked to poor oral hygiene and early-life ingestion of excessive fluoride, thereby resulting in dental fluorosis [13].
The millennium development goals (MDGs) aim at providing quality water to all Kenyans especially in rural setups by the year 2030. It, therefore, behooves the Kenyan Government and public institutions, for example, to find ways of reducing water fluoride concentration in areas where the risk of dental or skeletal fluorosis is prevalent. There are several methods of defluoridation of water available on the market. They include bone char, contact precipitation, Nalgonda, activated alumina, ion exchange, membrane filtration, nanofiltration, corn cob, and clay techniques [14]. Advanced treatment technologies are reverse osmosis (RO), electrodialysis, and distillation. Most of the water defluoridation technologies available in the Kenyan market are either expensive or unproven under local conditions. Other methods have seen minimum adoption because of a lack of practical information and experience.
One of these methods, the Nalgonda technique, which traces its origin to India, uses mainly two chemicals: Aluminium sulphate (alum) and lime (CaO). The technique initially saw some degree of success in two neighboring countries of Ethiopia and Tanzania. The method has, however, so far recorded minimum little traction or adoption in Kenya owing to the poor community management capacity and the breakdown in supply-chain of equipment and chemicals needed to run the process [15,16]. In a study by Dahi, et al. [17], conducted in Tanzania, it was estimated that a 20 L bucket of water to be defluorinated will consume 12.8 g alum and 6.4 g of lime to reduce fluoride from 12.5 and 8.8 mg L-1 to 2.1 ± 0.7 mg L-1. In Kenya, agriculture lime costs US$60 (Ksh. 6,600) per metric ton or US$0.00006 per g. To treat 20 liters of water will cost US$0.0004 per day for a family of 4 members, or US$0.016 per month. If one considers that the majority of extremely low-income people live in the countryside on less than US$1.90 per day [18], replacing a chemical with a freely available product should help reduce fluoride content in drinking water and promote the adoption of a modified Nalgonda technique.
One potential source of inexpensive or low-cost CaO is wood ash, a by-product of wood fuel combustion, which can be found in many homesteads and is readily available. In Kenya, wood fuel is the major form of biomass energy contributing 70% of the national energy demand and 90% of rural households’ energy requirements through the consumption of firewood or charcoal [19]. With an estimated 40 million inhabitants (10 million families) living in the countryside, Kenya produces on average 100 kg of wood ash per family per year or 1.0 million metric tons of ash per year [20,21]. This large amount of ash can be the source of a low-cost CaO and provide adequate leachate for defluoridation needs.
Wood ash has been successfully applied in agriculture as a soil amendment and a liming agent [22-24]. Studies have shown that wood ash can contain in some cases close to 80% CaO [25]. Wood ash leachate has also been tested as a coagulant and as a supportive electrolyte to remove the color from industrial wastewater [26]. Consequently, in this study, we explore the possible replacement of one of the chemicals, CaO, with wood ash leachate, as a co-precipitation agent with alum in the defluoridation process. Successful substitution of CaO will alleviate the burden of the chemical supply chain and assist in the adoption and sustainability of the modified Nalgonda technique of defluoridation.
Study area
The water samples were collected from the Lake Bogoria area in Baringo County, Fluorspar Kenya in Elgeyo Marakwet county, and the Karachuonyo area (the source of hot springs) in Homa Bay County. The area around Lake Bogoria has an altitude of about 1,700 meters above sea level and lies at a latitude of 0° 28´N and a longitude of 35° 58´E. The rainfall around lake Bogoria varies between 30 mm to 145 mm and a temperature of 11 to 28 oC. Karachuonyo is located in Western Kenya, on the shores of Lake Victoria at an altitude of about 1280 meters above sea level. The area lies at a latitude of 0° 26’ 60’’ N and a longitude of 34° 37’ 60’’ E. At Karachunyo, the annual rainfall is between 250 mm and 800 mm and the temperature varies from 14 oC to 35 oC. Fluorspar Kenya sits at an elevation of 1,000 meters in the Great Rift Valley and lies at a latitude of 0°18’37.9”N and a longitude of 35°38’07.9”E. Annual temperature at Fluorspar Kenya area vary between 14 °C to a maximum of 24 oC, and rainfall 400 mm to 1400 mm per annum.
Water samples
Water samples were collected from Karachunyo, Baringo, and Fluorspar Kenya with fluoride concentrations ranging from 91 mg L-1, 9.1 mg L-1 to 5.1 mg L-1 respectively. The areas chosen are known for their high fluoride concentration levels. Wood ash, sourced locally from a food processing plant was sieved using a 35-mesh screen to remove any un-combusted material and obtain ash particles of less than 1.0 mm, which were used in all the experiments. A portion of the ash was acid-digested [27] and analyzed for its chemical content using an inductively coupled plasma mass spectrometer (ICP-MS). Another portion was used to prepare the leachate by dissolving one part of wood ash (bone dry weight) into two parts of distilled water (1:2 ash: water), stirred overnight, and decanted to obtain a clear supernatant. Five volumes of raw water were prepared: 100, 200, 300, 400, and 500 ml. Several alum solutions were also prepared: 1%, 2%, 3%, and 4%, to be used on the raw water with a fluoride concentration of 5.1 mg L-1 and 9.1 mg L-1. Stronger alum solutions i.e. 5%, 6%, 7%, 8% and 9% were used for water samples containing 91 mg L-1 of F-. Jar tests were run to dose alum in fluoride-contaminated waters. Wood ash leachate was then added to the treated water to adjust the pH to 7. The treated water samples were analyzed for fluoride concentration by the SPADNS spectrophotometric method. Total coliforms using the plate count method were determined in selected water samples. Turbidity was measured by the HACH DR 4000 Spectrophotometer while total dissolved solids (TDS) were analyzed by gravimetric method. Selected water samples were analyzed by an ICP-MS (X Series 2, Thermo Scientific, USA) before and after treatment by the Homa method to determine the net impact of the technique on the water chemical concentration.
The experiment was set up as a factorial design where, the dependent variable was the final F- concentration of the treated water, while the different factors were the initial volume of raw water, the percentages of alum, and the volume of alum and wood ash leachate. Each test was run in triplicate. An analysis of variance (ANOVA) was done to determine the effect of alum and wood ash leachate and their potential interaction on the fluoride removal from the raw water in each site. In addition, a multivariate model was fitted in each region to determine factors significantly influencing the residual or final fluoride levels.
Defluoridation was achieved within three to five minutes during which a gelatinous precipitate was observed. The wood ash aided double precipitation method reduced fluoride levels significantly in all three sets of water samples. Analysis of variance showed significant effects of alum concentration and Wood leachate on residual fluoride level.
a) Removal efficiency
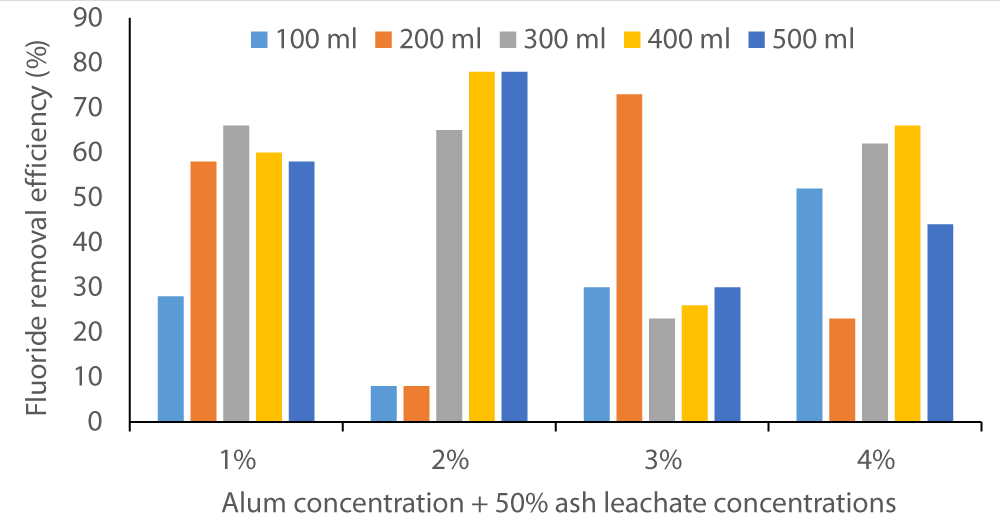
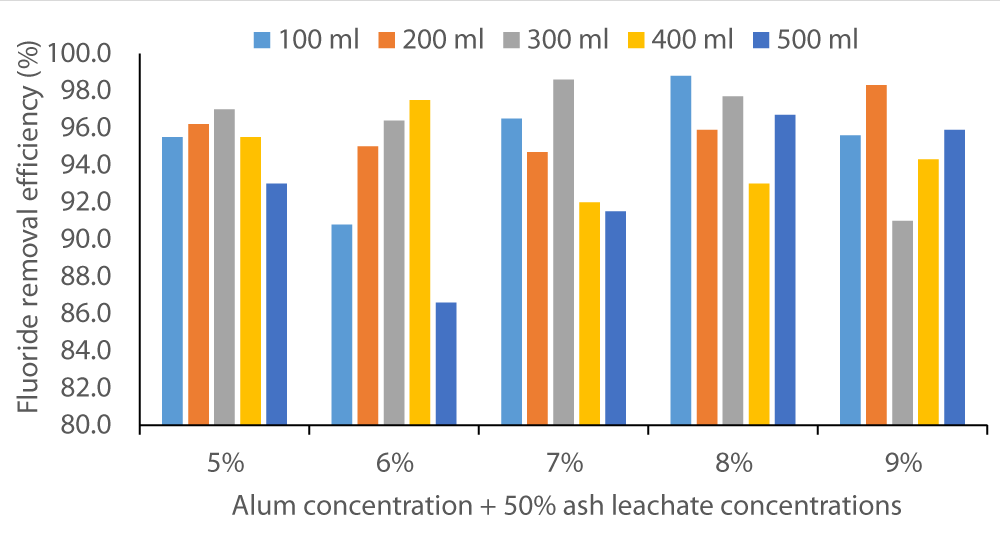
The two figures below (Figure 2 and Figure 3) show that co-precipitation of F- occurred at various levels of alum concentration and wood ash leachate volumes. The fluoride removal efficiency started as below 10% in Baringo water and rose to almost 80%. In Karachunyo water samples, the removal efficiency varied between 86% to 99% (Figure 3).
Figure 2: Fluoride removal efficacy by co-precipitation method on water samples from Baringo using 1% to 4% alum solutions in 100 to 500 sample volumes.
Figure 3: Fluoride removal efficiency by co-precipitation method on water samples from Karachuonyo using 5% to 9% alum solutions in 100 to 500 sample volumes.
This suggests that the Homa method efficiency is site dependent. All the water samples may not require the same dosage and, hence, prior testing/trial is necessary before this method is fully implemented. As Orori, et al. [26] determined in their study, wood ash can be an effective coagulant for various wastewater treatments (color removal) where ion precipitation is required, used alone or in combination with other coagulants.
b) Volumes of ash leachate and alum used
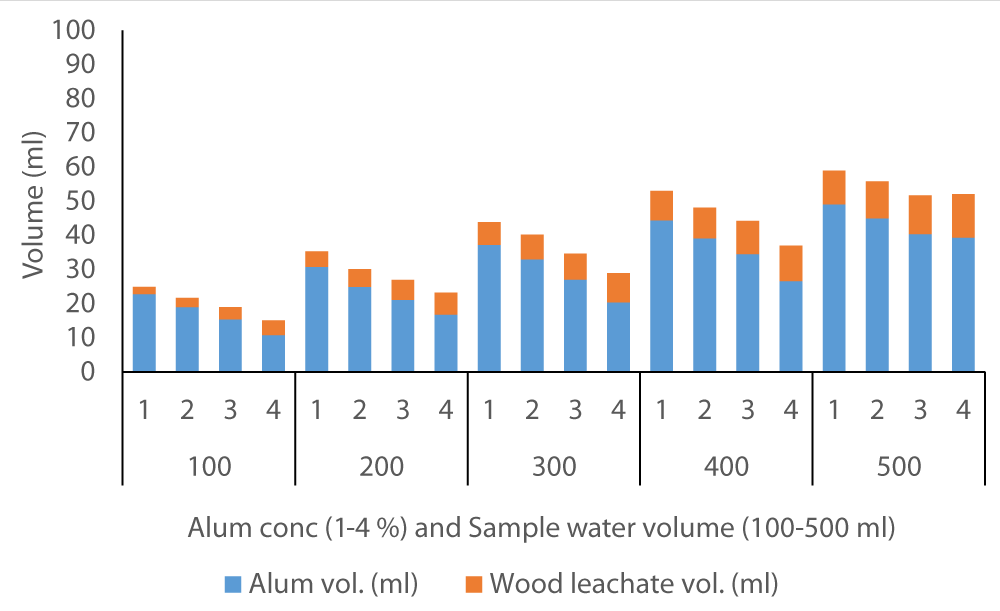
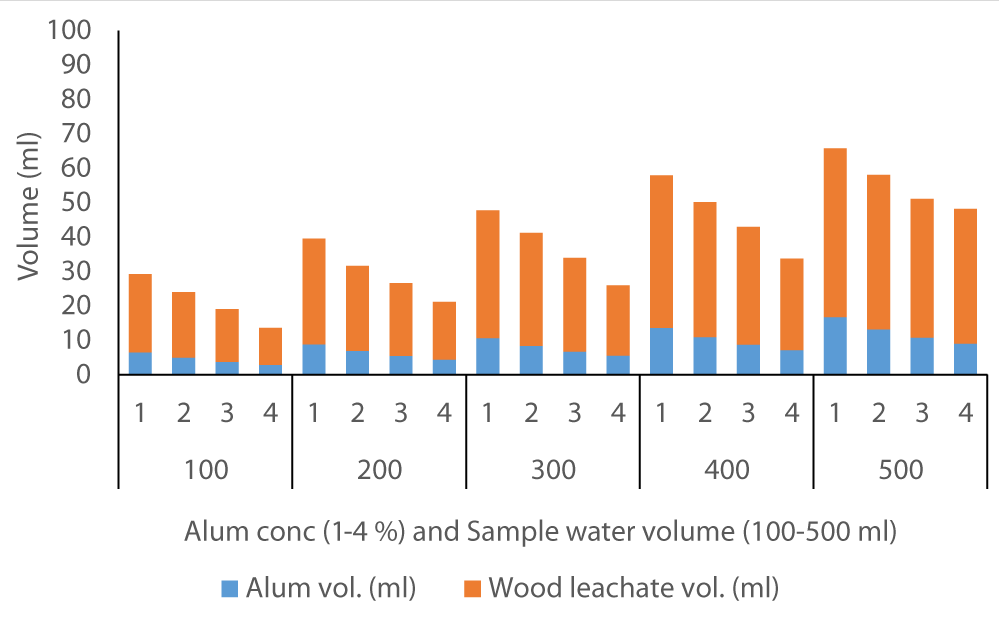
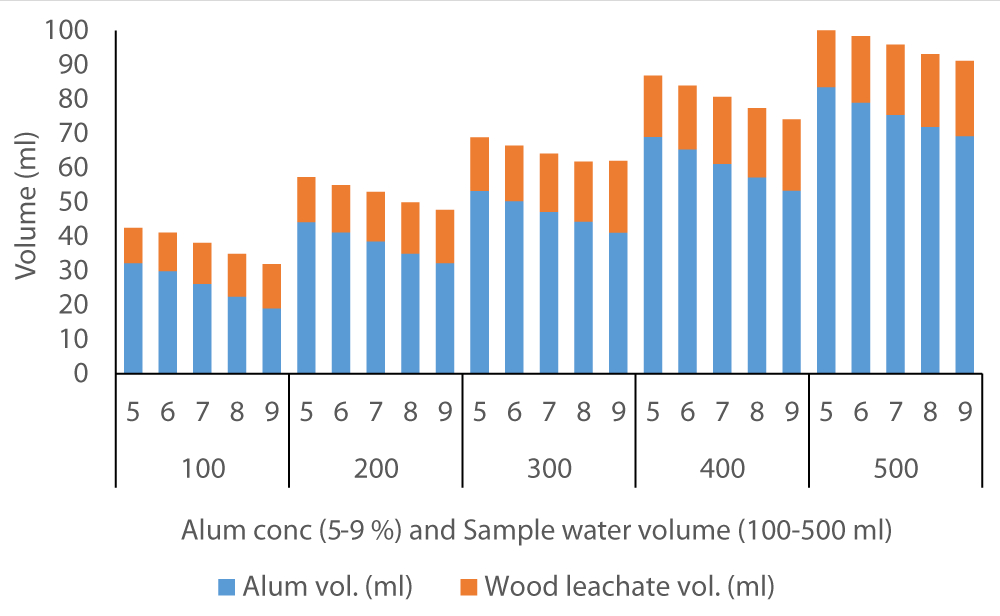
The average volume of wood ash leachate (7.35 ml) used to achieve neutral pH in Fluorspar (Figure 4) samples was lower than in Baringo (28.84 ml) as the initial F- was much higher in Baringo (Figure 5). This was likely attributed to the buffering capacity of Fluorspar water, and difference in other properties of the water samples from the two areas. In the case of Karachuonyo, the volumes of alum were much higher (Figure 6) due to their initial high F- content (91 mg L-1). The proportion of wood ash leachate required to attain neutral pH seemed to be lower for Fluorspar (0.26:1) as compared to Karachuonyo (0.37:1) but far much higher for Baringo (3.71:1)
Figure 4: Volumes of ash leachate and alum used to neutral pH in Fluorspar water.
Figure 5: Volumes of ash leachate and alum used to neutral pH in Baringo water.
Figure 6: Volumes of ash leachate and alum used to neutral pH in Karachuonyo water.
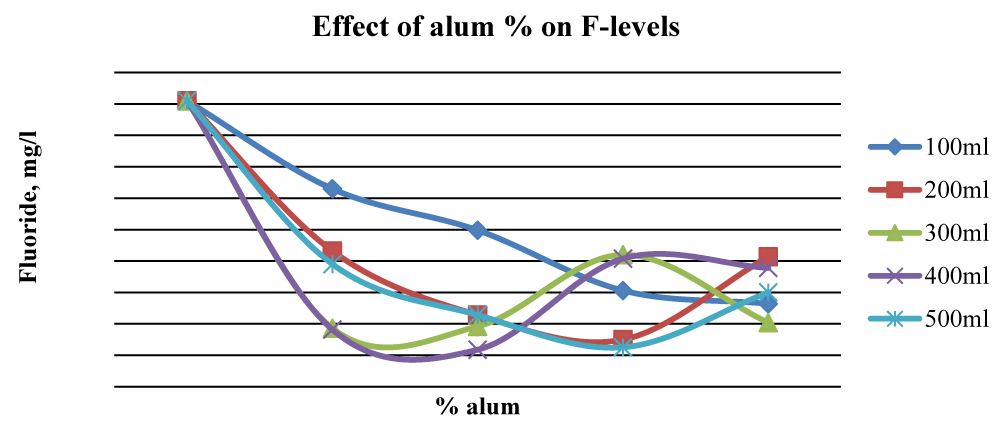
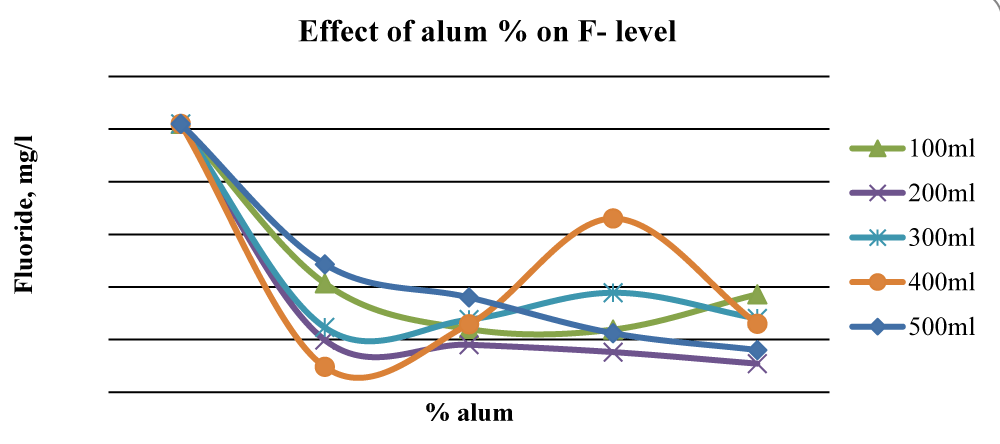
For Baringo samples, there was an overall significant reduction (p < 0.05) of fluorides once ash leachate and alum were added up to 2% alum. At 2% alum concentrations, 400 ml gave the best results while at 3% alum, 200 ml and 500 ml water gave substantial reduction levels of F- in Baringo water (Figure 7), and the differences between initial and final F- concentration were statistically significant (p < 0.05).
Figure 7: Effect of alum concentration on F- levels in Baringo water.
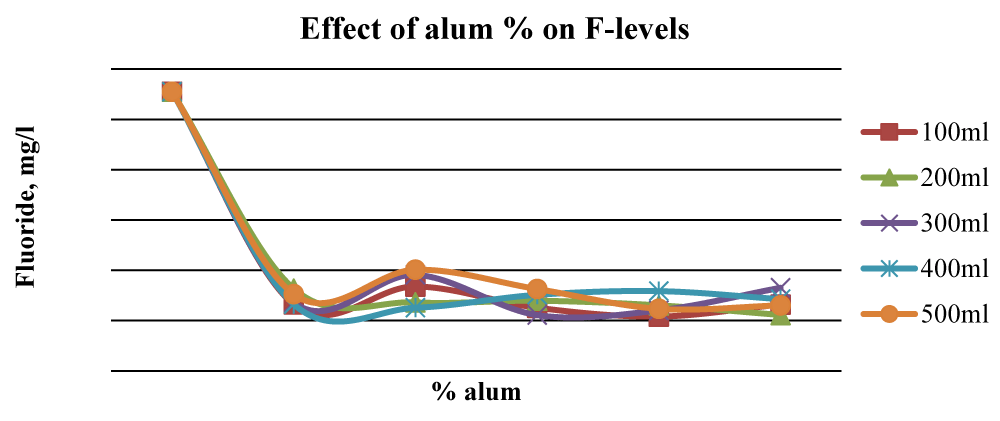
This was attributed to the combined stoichiometric reaction between alum and other divalent/trivalent ions (Ca2+, Fe2+, Al3+, Fe3+) present in the wood ash leachate with F- ions. Figure 7 shows that beyond 2% alum, the results of Fluoride removal were mixed, although, at 4% alum, the final F- concentration was much lower than the initial value. The initial fluoride levels in Karachuonyo water samples were quite high at 91 mg L-1 (Figure 6). However, the combined addition of ash leachate and alum significantly reduced fluoride levels (p < 0.05) to below 13 mg L-1 at 5% alum concentration, and the reduction appeared steadier compared to that F- reduction in the other two areas (Figure 8). The 200 ml water sample had a gradual reduction of F- and gave the best results attaining residuals of 2.2 mg L-1 at 9% alum concentration.
Figure 8: Effect of alum concentration on F- levels in Karachuonyo water.
Samples from Fluorspar had an initial fluoride level of 5.1 mg L-1 (Figure 9). There was a general reduction of fluorides with the addition of ash leachate and alum at all concentrations. The 200 ml sample gave the best result, however, at 4% alum, all the water samples had fluoride levels below 2.0 mg L-1. The 400 ml water sample from Fluorspar did not exhibit a specific trend. This could be attributed to the mishandling of the experimental water samples from this area.
Figure 9: Effect of alum concentration on Fluoride levels in Fluorspar water.
C) Effect of wood ash leachate
The results of the wood ash analysis are presented in Table 1. The major chemical elements determined in wood ash were Ca, followed by K, Mg, Si, P, Al, Mn, Fe, Na, and Zn. We theorize that in solution, alum, a well-known universal coagulant normally requires an alkaline range (7.0 to 8.5) to produce effective coagulation flocs. Heavy dosages of alum will normally depress the pH and impart non-carbonate hardness in water. The application of wood ash leachate probably brought the necessary alkalinity to the water and helped destabilize F-. In addition, most of the elements found in wood ash (Table 1) yielded divalent or trivalent counter ions that acted also as a coagulant, thereby contributing to further precipitation of F- from the raw water. Wood ash has been shown in previous studies to be a good low-cost fertilizer and liming agent [22,28]. The leachate was also successfully used to remove the color from a Kraft pulp mill effluent [26] confirming its capability to act as a coagulant.
| Table 1: Chemical analysis of wood ash sample in triplicate. | |||
| Compound / Element | Concentration in μg/g | ||
| Aluminium | 10,415 | 12,825 | 13,115 |
| Antimony | 264 | 142 | 65 |
| Arsenic | <20 | <20 | <20 |
| Barium | 1,301 | 1,490 | 1,640 |
| Beryllium | <5 | <5 | <5 |
| Bismuth | <30 | <30 | <30 |
| Cadmium | <2 | <2 | <2 |
| Calcium | 187,480 | 217,480 | 241,180 |
| Cerium | <40 | <40 | <40 |
| Chromium | 52 | 37 | 49 |
| Cobalt | <3 | <3 | <3 |
| Copper | 345 | 620 | 588 |
| Iron | 8,796 | 11,951 | 9,981 |
| Lanthanum | <10 | <10 | <10 |
| Lead | 51 | 75 | 200 |
| Lithium | 35 | 55 | 70 |
| Magnesium | 59,730 | 68,060 | 76,970 |
| Manganese | 10,549 | 11,789 | 13,489 |
| Molybdenum | 18 | 21 | 26 |
| Nickel | 59 | 64 | 151 |
| Phosphorus | 16,950 | 19,060 | 21,720 |
| Potassium | 110,500 | 86,590 | 47,720 |
| Selenium | <50 | <50 | <50 |
| Silicon | 33,867 | 39,77 | 42,647 |
| Sodium | 2,961 | 4,947 | 4,365 |
| Titanium | 780 | 1,060 | 1,012 |
| Vanadium | 13 | 18 | 23 |
| Ytterbium | 3 | 3 | 3 |
| Zinc | 2,678 | 2,139 | 1,001 |
| Zirconium | 14 | 14 | 16 |
| Carbonate | 62.8 | 60.0 | 58.4 |
In addition to the removal or reduction of F-, wood ash leachate could be responsible for raising the pH of the treated water, perhaps making the co-precipitation more effective. Possible precipitation reactions of wood ash leachate are presented in the section below. It was also observed that defluoridation with the Homa method yielded a substantial amount of sludge deposit at the end of the experiment, which may require proper handling and disposal.
d) Possible chemical reaction during the Homa process
Multiple co-precipitations take place during the Homa process. The following equations represent possible combinations/reactions that can help explain the precipitation and the removal of fluoride ions. We only considered wood ash elements with concentrations above 1000 µg g-1.
Proposed Aluminium precipitation of Fluoride ions (Acidic medium):
(1)
Co-precipitation (non-stoichiometric, undefined product):
complex + undefined product (2)
pH adjustment:
complex (3)
(4)
(5)
Precipitation of calcium fluoride:
(6)
Precipitation of fluorapatite:
(7)
(8)
Or (9)
(10) is a gas.
(11) KF is a solid that requires drastic conditions for its formation.
(12) this is a crystalline powder slightly soluble in water.
(13) used in the fluoridation of water
Manganese trifluoride MnF4, MnF2 (a pink solid), and MnF3 may have been formed during fluoride removal. Silicon tetrafluoride (SiF4) which is a gas at room temperature, probably was released into the atmosphere. However, combinations or reactions between fluoride ions and phosphorus, for example, which require different pathways and more drastic conditions for their formation, may not have been present during the defluoridation process.
e) General findings
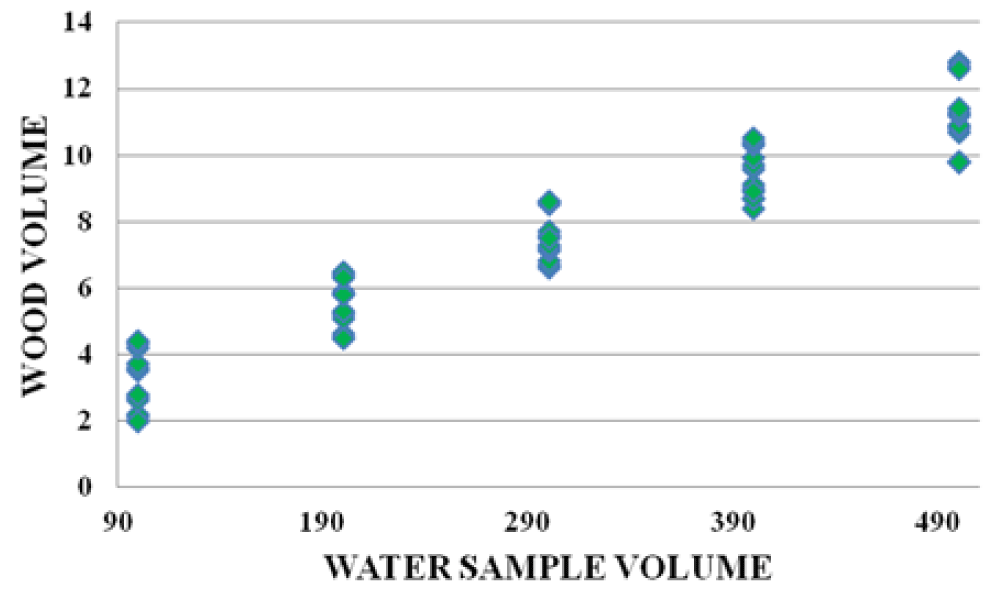
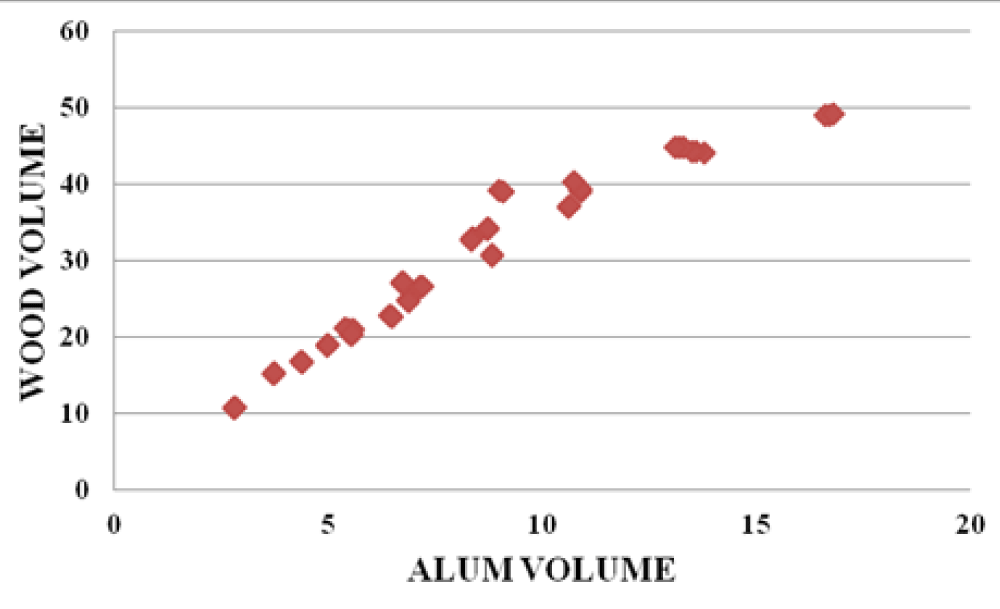
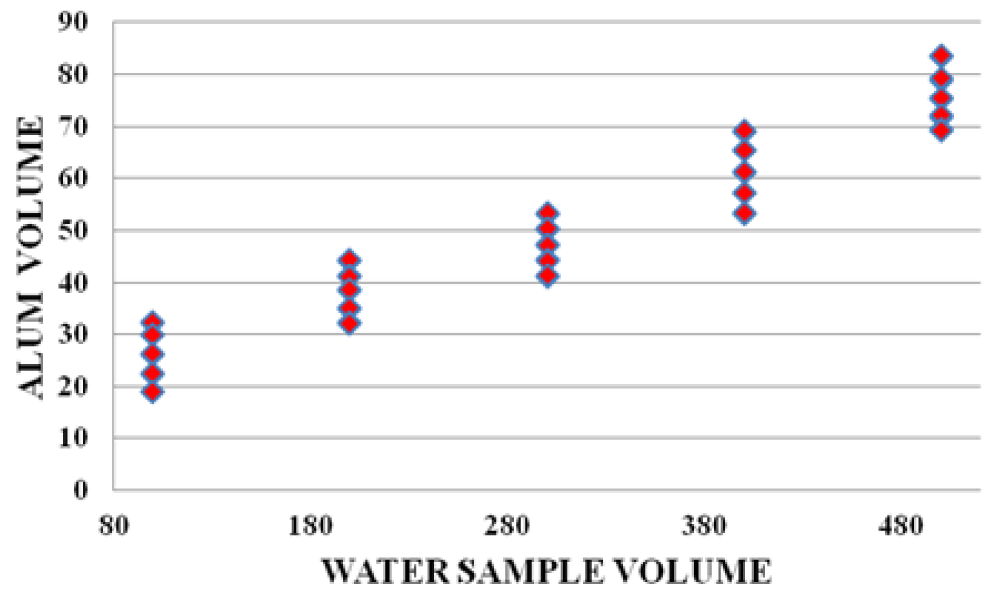
There were strong relationships between wood leachate volume versus water sample volume, Alum volume versus wood leachate volume, and water sample volume vs Alum volume. The graphs below (Figures 10-12) are samples of the observed trends.
Figure 10: Water Sample volume vs Wood volume for Fluorspar area.
Figure 11: Alum volume vs Wood volume for the Baringo area.
Figure 12: Water sample volume vs Alum volume for Karachuonyo area.
f) Statistical analysis
A multivariate analysis was used to determine relationships between the residual or final fluoride concentration (dependent variable) and the various factors i.e. the alum concentration, alum volume, and the wood ash leachate volume.
Analysis of variance (ANOVA)
ANOVA results showed significant differences for water sample volume (F0.05,4,50 = 28,927.2; p < 0.0001), Alum concentration (F0.05,4,50 = 35,652.0; p < 0.0001) and their interaction (F0.05,16,50 = 35,652.0; p < 0.0001) in Karachuonyo. Similar significant differences were also found in Fluorspar and Baringo. The separation of means for Karachuonyo showed that there were significant differences among all 5 water sample volumes. Similar results were reported for alum concentrations where significant differences were found among all 5 levels Tables 2,3.
| Table 2: Tukey grouping for Water Sample volumes. | ||
| Tukey Grouping | Mean | Water Sample Volumes |
| A | 12.90400 | 6 |
| B | 7.91333 | 7 |
| C | 6.95867 | 9 |
| D | 6.54800 | 5 |
| E | 5.15933 | 8 |
| Means with the same letter are not significantly different. | ||
| Table 3: Tukey grouping for Alum concentration. | ||
| Tukey Grouping | Mean | Water Sample Volumes |
| A | 11.03600 | 400 |
| B | 10.54600 | 500 |
| C | 6.57400 | 100 |
| D | 5.75600 | 200 |
| E | 5.57133 | 300 |
| Means with the same letter are not significantly different. | ||
A multivariate regression model of the following general form was proposed:
Where;
Y = Residual fluoride in water samples
X = Wood Leachate volume,
W = Alum Concentration,
X*W = Interaction and a, b, g d are regression coefficients, and
ε = Error term
The model was fitted to elucidate the relationship between residual or final fluoride concentration, wood leachate, and alum concentration and whether there existed an interaction between alum concentration and wood leachate.
The results showed that it was only in Baringo that the interaction term was significant. The model for Baringo was: α = 7.9022 β = -0.1268 γ = -1.2920 and δ 0.0303 and p - value = 0.0038. This, therefore, confirms that there was a strong interaction between wood ash leachate and alum, indicating how strong the two chemicals can work in tandem to reduce fluoride concentration in water. Although the interaction in the other two group water samples (Fluorspar and Karachuonyo) was not statistically significant as with the case of Baringo, it is safe to assume that wood ash leachate may have helped reduce fluoride in these water samples as well.
g) Total dissolved solids (TDS)
The removal of fluoride from water using the Homa method had a negative effect on total dissolved solids (TDS) as reported in Table 4. The final concentration of TDS increased probably as a result of the introduction of the wood ash leachate compound. The application of the Homa method will therefore necessitate further treatment to reduce this substantial amount of chemicals and other dissolved solids. The impact on TDS could be reduced by introducing a filtration step before any domestic consumption is envisaged.
| Table 4: TDS results of treated water after reduction of fluoride. | |||||
| Baringo Water | Karachuonyo Water | ||||
| Volume of water ml |
Initial | Final | Volume of water ml |
Initial | Final |
| TDS (mg L-1) | TDS (mg L-1) | TDS (mg L-1) | TDS (mg L-1) | ||
| 100 | 558 | 5600 | 100 | 18 | 18900 |
| 100 | 558 | 6300 | 100 | 18 | 19600 |
| 100 | 558 | 7000 | 100 | 18 | 19600 |
| 200 | 558 | 4900 | 200 | 18 | 22400 |
| 200 | 558 | 5600 | 200 | 18 | 21700 |
| 200 | 558 | 5600 | 200 | 18 | 15500 |
| 300 | 558 | 9100 | 300 | 18 | 20300 |
| 300 | 558 | 3500 | 300 | 18 | 21700 |
| 300 | 558 | 5600 | 300 | 18 | 22400 |
h) Homa method effect on turbidity and coliform count
The Homa method gave mixed results on turbidity. For example, as presented in Table 5, whereas Karachuonyo’s treated water recorded a slight upswing in its turbidity, the results on water from Baringo with an initial higher value yielded lower final turbidity after treatment, sometimes well below the 5 NTU recommended by World Health Organization (WHO), and the difference was statistically significant (p < 0.05). Turbidity from Fluorspar water was equally lowered from 35 NTU to below 15 NTU.
| Table 5: Turbidity results of the water before and after treatment. | ||||||
| Baringo Water | Karachuonyo Water | |||||
| Volume of water ml |
Initial turbidity (NTU) |
Final turbidity (NTU) |
Volume of water ml |
Initial turbidity (NTU) | Final turbidity (NTU) |
|
| 100 | 12.7 | 1.9 | 100 | 3.0 | 5.2 | |
| 100 | 12.7 | 9.2 | 100 | 3.0 | 5.0 | |
| 200 | 12.7 | 2.0 | 200 | 3.0 | 4.6 | |
| 200 | 12.7 | 2.3 | 200 | 3.0 | 15.4 | |
| 200 | 12.7 | 8.3 | 200 | 3.0 | 3.2 | |
Total coliform counts L-1 in samples of water from Baringo, Fluorspar, and Karachunyo were considerably reduced from 290, 270, and 310 to zero respectively. Although limited in the number of water samples tested, the results from this study indicate that no expensive disinfection method might be required after fluoride reduction using the Homa technique.
The ability of wood ash to effectively disinfection of wastewater is well documented [29,30]. For example, Ivanković, et al. [30] conducted a study on urban wastewater and landfill leachate and found that the addition of ash at a concentration of 10 g L⁻¹ (1%) inactivated bacterial populations in the wastewater and the removal of faecal coliforms and intestinal enterococci after 6 h of contact were 100% (below the detection limit; < 1 CFU mL-1). In another study, the application by Kocaer, et al. [29] of fly ash in conjunction with a minimum amount of quicklime on wastewater sludge led to an effective faecal coliform removal in both alkaline stabilization and pasteurization processes of the sludge. It is equally worth mentioning the effectiveness of ash in reducing bacterial counts on hands during handwash programs in different studies conducted in Bangladesh [31,32] and India [33]. These results corroborate the potential benefit of defluoridation of water with the Homa method through disinfection.
The final treated water exhibited a substantial amount of mineral or chemical addition as shown in Table 6. When compared to raw water samples (BRO), the treated ones in BR1 to BR5 showed a sizeable increase in Mg (2411%), Ca (7929%), and Al (6275%) concentrations at BR4 confirming a chemical enrichment from the Homa method. This could be a pointer to the Homa technique making the treated water slightly hard. The results from this corroborate observations by Etiegni & Campbell [34] when they studied ash solubility in water.
| Table 6: Chemical composition of water sample from Baringo before and after treatment. | |||||||||||
| The concentration of chemical elements µg L-1* | |||||||||||
| BRO | BR5 | BR4 | BR2 | BR1 | BRO | BR5 | BR4 | BR2 | BR1 | ||
| Na | 6040000 | 214000 | 1162200 | 234200 | 5186000 | Co | < 2 | < 2 | < 2 | < 2 | < 2 |
| Mg | 622 | 1492 | 15002 | 1708 | 426 | Ni | < 2 | < 2 | < 2 | < 2 | < 2 |
| Al | 156.2 | 304 | 9802 | 964 | 1136 | Cu | < 2 | < 2 | 1344.2 | 116.8 | < 2 |
| K | 125200 | 2800000 | 486200 | 3756000 | 5214000 | Zn | 47 | 99.6 | 4888 | 130.6 | 41 |
| Ca | 4820 | 7500 | 382200 | 13100 | 1956 | Mo | 146.6 | 90.8 | < 2 | 116 | 570 |
| V | < 2 | < 2 | < 2 | < 2 | < 2 | Cd | < 2 | < 2 | < 2 | < 2 | < 2 |
| Cr | < 2 | < 2 | < 2 | < 2 | < 2 | Ba | 142 | < 2 | < 2 | 84.8 | < 2 |
| Mn | < 2 | 42.2 | < 2 | 60.4 | < 2 | Pb | < 2 | < 2 | < 2 | < 2 | < 2 |
| Fe | 106 | 127.6 | 4862 | 141.4 | 52.6 | ||||||
| *BR5 = Baringo water sample (500 ml) treated with 1% Alum *BR2 = Baringo water sample (200 ml) treated with 1% Alum *BR4 = Baringo water sample (400 ml) treated with 1% Alum *BR1 = Baringo water sample (100 ml) treated with 1% Alum *BRO = Raw water (untreated) from Baringo |
|||||||||||
This study has demonstrated that the Homa technique can be used to reduce fluoride (almost 99% in some cases) from highly fluoridated waters under 10 minutes of mixing. The Homa technique reduced the fluoride level below the maximum 1.5 mg L-1 recommended by WHO. Because of the availability of wood ash, a by-product of energy consumption in rural settings in Kenya, the method should help reduce CaO dependence by US$0.016 per month, for a section of the population living on less than US$1.90 per day and promote the adoption of this modified Nalgonda method of defluoridation. The new technique presented several advantages in addition to the reduction of F-. It provided some level of disinfection (reduction of coliform from 310 count/L to zero) and reduced turbidity (from 12.7 to 2.3 NTU in some cases) of the water. However, the observed mineral enrichment of the treated water (Ca in treated has increased by 7929%) and the production of a substantial amount of sludge could be a cause for concern that requires further investigation. It was recommended that more studies be carried out to determine the optimum ratios of wood ash leachate and alum needed to provide good quality water to the rural poor. Further extension of this research should look at making inexpensive filtration methods to remove any remaining suspended solids from the treated water.
We wish to acknowledge the assistance of Eldoret Water and Sanitation Company (ELDOWAS) for water analysis and University of Eldoret for providing the laboratory facility to carry out this project.
- Gevera P, Mouri H. Natural occurrence of potentially harmful fluoride contamination in groundwater: an example from Nakuru County, the Kenya Rift Valley. Environ Earth Sci. 2018; 77: 365.
- Mwiathi NF, Gao X, Li C, Rashid A. The occurrence of geogenic fluoride in shallow aquifers of Kenya Rift Valley and its implications in groundwater management. Ecotoxicol Environ Saf. 2022 Jan 1;229:113046. doi: 10.1016/j.ecoenv.2021.113046. Epub 2021 Dec 4. PMID: 34875514.
- Olaka LA, Wilke FD, Olago DO, Odada EO, Mulch A, Musolff A. Groundwater fluoride enrichment in an active rift setting: Central Kenya Rift case study. Sci Total Environ. 2016 Mar 1;545-546:641-53. doi: 10.1016/j.scitotenv.2015.11.161. Epub 2016 Jan 8. PMID: 26775113.
- Rusiniak P, Klaudia Sekuła K, Sracek O, Stopa P. Fluoride ions in groundwater of the Turkana County, Kenya, East Africa. Acta Geochim. 2021; 40(6): 945–960.
- Moturi Nyaora KW, Tole PM, Davies CT. The Contribution of Drinking Water towards Dental Fluorosis: A case study of Njoro Division, Nakuru District, Kenya. Environmental Geochemistry. 2002; 24(2): 123-130.
- Wambu EW, Agong SG, Anyango B, Akuno W, Akenga T. High fluoride water in Bondo-Rarieda area of Siaya County, Kenya: a hydro-geological implication on public health in the Lake Victoria Basin. BMC Public Health. 2014 May 17;14:462. doi: 10.1186/1471-2458-14-462. PMID: 24884434; PMCID: PMC4049395.
- Nair RK, Manji F. Challenges in African Hydrology and Water Resources. Proceedings of the Harare Symposium. July 1AHS. 1984;144.
- Francisca MM, Patrick CK, Peter GN. Assessment of the Impact of Groundwater Fluoride on Human Health: A Case Study of Makindu District in Kenya. Journal of Earth Science & Climatic Change. 2017; 8: 4. 1-7.
- Gevera P, Mouri H, Maronga G. Occurrence of fluorosis in a population living in a high-fluoride groundwater area: Nakuru area in the Central Kenyan Rift Valley. Environ Geochem Health. 2019 Apr;41(2):829-840. doi: 10.1007/s10653-018-0180-2. Epub 2018 Sep 1. PMID: 30173366.
- Edmunds WM, Smedley PL. Fluoride in natural waters. Essentials of Medical Geology. 2013;pp. 311-336.
- Ibrahim M, Asim Rasheed SM, Prabhakar P. Effects of fluoride contents in ground water: A review. International Journal of Pharmaceutical Applications. 2011; 2(2): 128-134.
- Jha SK, Mishra VK, Sharma DK, Damodaran T. Fluoride in the environment and its metabolism in humans. Rev Environ Contam Toxicol. 2011;211:121-42. doi: 10.1007/978-1-4419-8011-3_4. PMID: 21287392.
- Menya D, Maina SK, Kibosia C, Kigen N, Oduor M, Some F, Chumba D, Ayuo P, Middleton DRS, Osano O, Abedi-Ardekani B, Schüz J, McCormack VA. Dental fluorosis and oral health in the African Esophageal Cancer Corridor: Findings from the Kenya ESCCAPE case-control study and a pan-African perspective. Int J Cancer. 2019 Jul 1;145(1):99-109. doi: 10.1002/ijc.32086. Epub 2019 Jan 12. PMID: 30582155; PMCID: PMC6519293.
- Lavanya HD, Madhushree C, Vani AM. Defluoridation of groundwater using corn cobs powder. Int J Eng Sci. 2017; 6(60): 78-81.
- Brunson LR, Sabatini DA. An evaluation of fish bone char as an appropriate arsenic and fluoride removal technology for emerging regions. Environmental Engineering Science. 2009; 26(12): 1777-1784.
- Yami LT, Jim F, Chamberlain FJ, Beshah ZF, Sabatini AD. Performance enhancement of Nalgonda technique and pilot testing electrolytic defluoridation system for removing fluoride from drinking water in East Africa. African Journal of Environmental Science and Technology. 2018; 12(10): 357-369.
- Dahi E, Mtalo F, Njau B, Bregnhj H. Defluoridation using the Nalgonda Technique in Tanzania. Reaching the unreached: challenges for the 21st century. 22nd WEDC Conference New Delhi, India. 1996; 1-3.
- Faria J. People living in extreme poverty in Kenya 2016-20221, by area. 2022. Statista.
- Ministry of Energy Study on Kenya’s Energy Demand, Supply and Policy Strategy for Households, Small scale Industries and Service Establishments. 2002. Kamfor Consultants, Nairobi, Kenya.
- Statistical yearbook (Central Statistical Office). 2015.
- Simanowicz B, Becher M, Jaremko D, Skwarek K. Possibility for the uses of wood ashes in Agriculture. Journal of Ecological Engineering. 2018; 19: 191-196. doi.org/10.12911/22998993/86156.
- Huang H, Campbell GA, Folk R, Mahler LR. Wood ash as a soil additive and liming agent for wheat: Field studies. Communication in soil Science and Plant Analysis. 2008; 25-33.
- Pitman R. Wood ash use in forestry - A review of the environmental impacts. Forestry. 2006; 79(5): 563-588. DOI: 10.1093/forestry/cpl041.
- Arshad M, Soon KY, Azooz HR, Lupwayi ZN, Chang XS. Soil and Crop Response to Wood Ash and Lime Application in Acidic Soils. 2012. Agronomy Journal. 104(3): 71. DOI: 10.2134/agronj2011.0355.
- Sanusi MO, Komolafe DO, Ogundana OT, Olaleke OM, Sanni YY. Development of Wood-ash/Resin Polymer Matrix Composite for Body Armour Application. Journal of Engineering and Technology. 2016; 1:1-17.
- Orori OB, Etiegni L, Rajab SM, Situma ML, Ofosu-Asiedu K. Decolorization of a pulp and paper mill effluent in Webuye Kenya by a combination of electrochemical and coagulation methods. Pulp and Paper Canada. 2005; 106(3): 21-26.
- Okalebo RJ, Gathua WK, Woomer LP. Laboratory Methods for Soil and Plant Analysis: A Working Manual. 2002. TSBF, Nairobi.
- Etiégni L, Campbell GA, Mahler LR. Evaluation of wood ash disposal on agricultural land. I. Potential as a soil additive and liming agent. Communication in soil Science and Plant analysis. 2008; 243-256.
- Kocaer FO, Alkan U, Baskaya HS. Use of lignite fly ash as an additive in alkaline stabilisation and pasteurisation of wastewater sludge. Waste Manag Res. 2003 Oct;21(5):448-58. doi: 10.1177/0734242X0302100507. PMID: 14661892.
- Ivanković T, Hrenović J, Itskos G, Koukouzas N, Kovačević D, Milenković J. Alkaline disinfection of urban wastewater and landfill leachate by wood fly ash. Arh Hig Rada Toksikol. 2014 Dec;65(4):365-75. doi: 10.2478/10004-1254-65-2014-2546. PMID: 25720024.
- Hoque BA, Mahalanabis D, Pelto B, Alam MJ. Research methodology for developing efficient handwashing options: an example from Bangladesh. J Trop Med Hyg. 1995 Dec;98(6):469-75. PMID: 8544234.
- Hoque BA. Handwashing practices and challenges in Bangladesh. Int J Environ Health Res. 2003 Jun;13 Suppl 1:S81-7. doi: 10.1080/0960312031000102831. PMID: 12775383.
- Anuradha P, Yasoda Devi P, Prakash MS. Effect of handwashing agents on bacterial contamination. Indian J Pediatr. 1999 Jan-Feb;66(1):7-10. doi: 10.1007/BF02752341. PMID: 10798029.
- Etiégni L, Campbell GA. Physical and chemical characteristics of wood ash. Bioresource Technology. 1991; 37(2): 173-178.
- Manji F, Kapila S. Fluoride and fluorosis in Kenya-An overview. In: Likimani, S, editor. Fluorosis research strategies, Nairobi, Kenya: African Medical Research Foundation. 1984; 11-21.