Abstract
Research Article
Nano-silica from kaolinitic clay used as adsorbent for anionic and cationic dyes removal: linear and non-linear regression isotherms and kinetics studies
Gustave Tchanang, Chantale Njiomou Djangang*, Charles Fon Abi, Danie Laure Mbella Moukouri, Guillonnel Trésor Nyadjou Djabo, Jean Marie Kepdieu and Philippe Blanchart
Published: 24 May, 2022 | Volume 6 - Issue 1 | Pages: 008-018
The increasing occurrence of wastewaters associated with industrial development has begotten a permanent search for new and more efficient techniques for the removal of hazardous substances such as heavy metals and dyes. The use of natural and available resources to develop improved and sustainable commodities for this purpose remains crucial and is among promising emerging green technologies for water treatment. It offers the gradual shifting of hazardous industrial chemicals precursors to the abundant non-metallic mineral resources that receive an added value. This work investigated the uptake capacity by the adsorption process of methylene blue (MB) and azocarmine G (AG) onto nano-silica synthesized from kaolinite clay. The effects of contact time (0-30 min), the adsorbent dosage (5-100 mg), the initial pH of the solution (1-11 for MB and 1-7 for AG), and the initial dye concentration (5-50 mg/L) were studied. The selected conditions to carry out kinetic and isotherm adsorption experiments were: 15 mins, 20 mg, 11 for MB, 1.01 for AG, and 50 mg/L. Four adsorption isotherms and three kinetic models were used to model the adsorption data thanks to linear and non-linear regression methods. From the obtained results, the Freundlich isotherm model fitted well the adsorption phenomenon while the pseudo-second-order kinetic model described well the adsorption mechanism. Furthermore, the free energy of adsorption was similar for the two absorbents, 0.71 kJ, pointing physisorption as the dominant adsorption mechanism. The optimum MB and AG uptake were respectively 13.8 and 36.1 mg/g. Conclusively, the nano-silica represents a potentially viable and powerful adsorbent whose use could lead to a plausible improvement in environmental preservation.
Read Full Article HTML DOI: 10.29328/journal.acee.1001034 Cite this Article Read Full Article PDF
Keywords:
Nano-silica; Adsorption; Dyes; Isotherms/kinetic models; linear/nonlinear regression
References
- Mohammad IK. Adsorption of methylene blue onto natural Saudi Red Clay: isotherms, kinetics and thermodynamic Studies. Mater Res Express 2020; 7: 055507.
- Hajjaji W, Andrejkovicová A, Pullar RC, et al. Effective removal of anionic and cationic by kaolinite and TiO2/kaolinite composite. Clay Miner. 2016;51:19–27.
- Tan IA, Ahmad AL, Hameed BH. Adsorption of basic dye on high-surface-area activated carbon prepared from coconut husk: equilibrium, kinetic and thermodynamic studies. J Hazard Mater. 2008 Jun 15;154(1-3):337-46. doi: 10.1016/j.jhazmat.2007.10.031. Epub 2007 Oct 13. PMID: 18035483.
- Pavithra KG, Kumar PS, Jaikumar V, et al. Removal of colorants from wastewater: A review on sources and treatment strategies. J Ind Eng Chem. 2019;75:1-19.
- Mahmoud ME, Nabil GM, El-Mallah NM, et al. Kinetics, isotherm and thermodynamic studies of the adsorption of reactive red 195 A dye from water by modified Switchgrass Biochar adsorbent. J Ind Eng Chem. 2016;37:156–167.
- Jingfei L, Yue S, Shu W, et al. Synthesis, Property Characterization and Photocatalytic Activity of the Polyaniline/BiYTi Polymer Composite. Polymers. 2017;9(69): 2-3.
- Yu K, Xiaoping Z, Shaoqi Z. Adsorption of Methylene Blue in Water onto Activated Carbon by Surfactant Modification. Water. 2020;12:587.
- Karaca S, Gürses A, Açikyildiz M, et al. Adsorption of cationic dye from aqueous solutions by activated carbon. Microporous Mesoporous Mater. 2008;115: 376–382.
- Dias EM, Petit C. Owards the use of metal–organic frameworks for water reuse: a review of the recent advances in the field of organic pollutants removal and degradation and the next steps in the field. J Mater Chem. 2015;3: 22484-22506.
- Oturan M, Aaron JJ. Advanced Oxidation Processes in Water/Wastewater Treatment: Principles and Applications. A Review. Criti Rev Environ Sci Technol. 2014;44(23): 2577-2641.
- Regulska E, Rivera-Nazario D, Karpinska J et al. Zinc porphyrin-functionalized fullerenes for the sensitization of titania as a visible-light active photocatalyst: river water and wastewaters remediation. Molecules. 2019;24(6): 1-17.
- Yu S, Liu M, Ma M, et al. Impacts of membrane properties on reactive dye removal from dye/salt mixtures by asymmetric cellulose acetate and composite polyamide nanofiltration membranes. J Membr Sci. 2010;350: 83-91.
- Asghar A, Abdul Raman AA, Wan D, et al. Advanced oxidation processes for in-situ production of hydrogen peroxide/hydroxyl radical for textile wastewater treatment: A review. J Clean Prod. 2015;87: 826–838.
- Kordouli E, Bourikas K, Lycourghiotis A, et al. The mechanism of azo-dyes adsorption on the titanium dioxide surface and their photocatalytic degradation over samples with various anatase/rutile ratios. Catal Today 2015;252: 128–135.
- Xiang Y, Gao M, Shen T, et al. Comparative study of three novel organo-clays modified with imidazolium-based gemini surfactant on adsorption for bromophenol blue. J Mol Liquids. 2019;286.
- Fan S, Wang Y, Wang Z, et al. Removal of methylene blue from aqueous solution by sewage sludge-derived biochar: Adsorption kinetics, equilibrium, thermodynamics and mechanism. J Environ Chem Eng. 2017;5: 601–61.
- Wang G, Wang S, Sun Z, Zheng S, Xi Y. Appl. Clay Sci. 2017;148: 1-10.
- Wang W, Huang G, An C, et al. Adsorption of anionic azo dyes from aqueous solution on cationic gemini surfactant-modified flax shives: Synchrotron infrared, optimization and modeling studies. J Clean Prod. 2018;172: 1986-1997.
- Hailu SL, Nair BU, Mesfin RA, et al. Preparation and characterization of cationic surfactant modified zeolite adsorbent material for adsorption of organic and inorganic industrial pollutants. J Environ Chem Eng. 2017; 5: 3319–3329.
- Joseph L, Jun BM, Flora JRV, Park CM, Yoon Y. Removal of heavy metals from water sources in the developing world using low-cost materials: A review. Chemosphere. 2019 Aug;229:142-159. doi: 10.1016/j.chemosphere.2019.04.198. Epub 2019 May 3. PMID: 31078029.
- Mohamed MM. Acid dye removal: comparison of surfactant-modified mesoporous FSM-16 with activated carbon derived from rice husk. J Colloid Interface Sci. 2004 Apr 1;272(1):28-34. doi: 10.1016/j.jcis.2003.08.071. PMID: 14985019.
- Leng L, Yuan X, Zeng G, et al. Surface characterization of rice husk bio-char produced by liquefaction and application for cationic dye (Malachite green) adsorption. Fuel. 2015;155: 77–85.
- Abdel-Fattah TM, Mohamed TM, Mahmoud E, et al. Biochar from woody biomass for removing metal contaminants and carbon sequestration. J Ind Eng Chem. 2015;22: 103–109.
- Mubarik S, Saeeda A, Athar MM, et al. Characterization and mechanism of the adsorptive removal of 2,4,6-trichlorophenol by biochar prepared from sugarcane baggase. J Ind Eng. Chem. 2016;33: 115–121.
- Rafatullah M, Sulaiman O, Hashim R, Ahmad A. Adsorption of methylene blue on low-cost adsorbents: a review. J Hazard Mater. 2010 May 15;177(1-3):70-80. doi: 10.1016/j.jhazmat.2009.12.047. Epub 2009 Dec 14. PMID: 20044207.
- Pathania D, Sharma S, Singh P. Removal of methylene blue by adsorption onto activated carbon developed from Ficus carica bast. Arab J Chem. 2017;10: 1445-1451.
- Qu X, Alvarez PJ, Li Q. Applications of nanotechnology in water and wastewater treatment. Water Res. 2013 Aug 1;47(12):3931-46. doi: 10.1016/j.watres.2012.09.058. Epub 2013 Mar 26. PMID: 23571110.
- Bhuvaneswari R, Nagarajan V, Chandiramouli R. Red tricycle phosphorene nanoribbon as a removing medium of sulfadiazine and sulfamethoxazole molecules based on first-principles studies. J Mole Liquids. 2021;336: 116294.
- Abu Al-Rub FA, Fares MM, Mohammad AR. Use of nanohybrid nanomaterials in water treatment: highly efficient removal of ranitidine. RSC Adv. 2020 Oct 8;10(61):37050-37063. doi: 10.1039/d0ra05530a. PMID: 35521255; PMCID: PMC9057075.
- Manikandan S, Karmegam N, Subbaiya R, Karthiga Devi G, Arulvel R, Ravindran B, Kumar Awasthi M. Emerging nano-structured innovative materials as adsorbents in wastewater treatment. Bioresour Technol. 2021 Jan;320(Pt B):124394. doi: 10.1016/j.biortech.2020.124394. Epub 2020 Nov 10. PMID: 33220545.
- Zinatloo-Ajabshir S, Mortazavi-Derazkola S, Salavati-Niasari M. Nd2O3-SiO2nanocomposites: A simple sonochemical preparation, characterization and photocatalytic activity. Ultrason Sonochem. 2018 Apr;42:171-182. doi: 10.1016/j.ultsonch.2017.11.026. Epub 2017 Nov 21. PMID: 29429658.
- Şenol ZM, Gürsoy N, Şimşek S, Özer A, Karakuş N. Removal of food dyes from aqueous solution by chitosan-vermiculite beads. Int J Biol Macromol. 2020 Apr 1;148:635-646. doi: 10.1016/j.ijbiomac.2020.01.166. Epub 2020 Jan 18. PMID: 31958562.
- Ahmadian-Fard-Fini S, Ghanbari D, Amiri O, Salavati-Niasari M. Electro-spinning of cellulose acetate nanofibers/Fe/carbon dot as photoluminescence sensor for mercury (II) and lead (II) ions. Carbohydr Polym. 2020 Feb 1;229:115428. doi: 10.1016/j.carbpol.2019.115428. Epub 2019 Oct 3. PMID: 31826498.
- Tchanang G, Djangang CN, Abi CF, et al. Synthesis of reactive silica from kaolinitic clay: Effect of process parameters. Appl Clay Sci. 2021;207: 106087
- Altenor S, Carene B, Emmanuel E, Lambert J, Ehrhardt JJ, Gaspard S. Adsorption studies of methylene blue and phenol onto vetiver roots activated carbon prepared by chemical activation. J Hazard Mater. 2009 Jun 15;165(1-3):1029-39. doi: 10.1016/j.jhazmat.2008.10.133. Epub 2008 Nov 18. PMID: 19118948.
- Boumediene M, Benaïssa H, George B, et al. Effects of pH and ionic strength on methylene blue removal from synthetic aqueous solutions by sorption onto orange peel and desorption study. J Mater Environ Sci. 2018;9(6): 1700-1711.
- Sandotin LC. Abattement des phosphates des eaux usées par adsorption sur des géomatériaux constitués de Latérite, grès et schistes ardoisiers. Thèse en Co-Tutelle L’Université de Lorraine (UL) et de L’Université Nangui Abrogoua (UNA), 2014.
- Lekene RBN, Kouotou D, Ankoro NO, et al. Development and tailoring of amino-functionalized activated carbon based Cucumerupsi manni Naudin seed shells for the removal of nitrate ions from aqueous solution. J Saudi Chem Society. 2021;25: 101316.
- Kifuani KMA, Vesituluta P, Lopaka B, et al. Adsorption d’un colorant basique, Bleu de Méthylène, en solution aqueuse, sur un bioadsorbant issu de déchets agricoles de Cucumeropsis mannii Naudin. Int J Biol Chem Sci. 2018;12(1): 558-575.
- Kifuani KMA, Noki VP, Ndelo DPJ, et al. Adsorption de la quinine bichlorhydrate sur un charbon actif peu coûteux à base de la Bagasse de canne à sucre imprégnée de l’acide phosphorique. Int J Biol Chem Sci. 2012;6(3): 1337-1359.
- Ying Z. The application of TiO2 in the dye wastewater treatment. Dyeing Finishing Technol. 2005;27(11): 26-29.
- Jianhua J, Yun Li. Research on TiO2/UV-Fenton Catalytic Oxidation of Azocarmine G Dye Wastewater School of Civil Engineering & Architecture. Wuhan University of Technology Wuhan P R China. 2010;978(1): 4244-4713.
- Gnana K, Suggala V, King P. Equilibrium and thermodynamic studies of methylene blue biosorption from aqueous solution using Syzygium cumini L. J Environ Res Develop. 2014;8(04): 964-973.
- Madhavakrishman S, Manickavasagam K, Vasanthakumar R, et al. Adsorption of Crystal violet dye from aqueous solution using Ricinus communis pericarp carbon as adsorbent. E-J Chem. 2009;6(4): 1109-1116.
- Zhang L, Xu T, Liu X, Zhang Y, Jin H. Adsorption behavior of multi-walled carbon nanotubes for the removal of olaquindox from aqueous solutions. J Hazard Mater. 2011 Dec 15;197:389-96. doi: 10.1016/j.jhazmat.2011.09.100. Epub 2011 Oct 5. PMID: 22018864.
- Tabi GA, Lekene RBN, Kouotou D, et al. Non-linear modelling of the adsorption of Indigo Carmine dye from wastewater onto characterized activated carbon/volcanic ash composite. Arabian J Chem. 2022;15: 103515.
- Kumar KV, Porkodi K. Mass transfer, kinetics and equilibrium studies for the biosorption of methylene blue using Paspalum notatum. J Hazard Mater. 2007 Jul 19;146(1-2):214-26. doi: 10.1016/j.jhazmat.2006.12.010. Epub 2006 Dec 10. PMID: 17222969.
- Laximi GS, Ahmazzaman MD. Adsorption technique for the removal of phenolic compound from wastewater using low-cost natural adsorbents. Assam University J Sci Technol. 2010;5(2): 156-166.
- Nanseu-Njiki CP, Dedzo GK, Ngameni E. Study of the removal of paraquat from aqueous solution by biosorption onto Ayous (Triplochiton schleroxylon) sawdust. J Hazard Mater. 2010 Jul 15;179(1-3):63-71. doi: 10.1016/j.jhazmat.2010.02.058. Epub 2010 Feb 25. PMID: 20334968.
- Saeed A, Sharif M, Iqbal M. Application potential of grapefruit peel as dye sorbent: kinetics, equilibrium and mechanism of crystal violet adsorption. J Hazard Mater. 2010 Jul 15;179(1-3):564-72. doi: 10.1016/j.jhazmat.2010.03.041. Epub 2010 Mar 16. PMID: 20381962.
- Arivoli S, Nandhakumar V, Saravanan S, et al. Adsorptiondynamics of copper ion by low cost activated carbon. Arabian J Sci Eng. 2009;34(1A): 112.
- Sugana M, Siva KN, Venkata SM, et al. Removal of divalent manganese from aqueous solution using Tamarindus indica fruit nut Shell. J Hazard Mater. 2010;2(1): 7-20.
- Şenol ZM, Gürsoy N, Şimşek S, Özer A, Karakuş N. Removal of food dyes from aqueous solution by chitosan-vermiculite beads. Int J Biol Macromol. 2020 Apr 1;148:635-646. doi: 10.1016/j.ijbiomac.2020.01.166. Epub 2020 Jan 18. PMID: 31958562.
- Hakan D, ˙Ilknur D, Fatma T, et al. Adsorption of chromium(VI) from aqueous solution by activated carbon derived from olive bagasse and applicability of different adsorption models. Chem Eng J. 2008;144: 188-196.
- Aljamali, NM, Aldujaili, R AB, Alfatlawi, IO. Physical and chemical adsorption and its applications. Int J Thermodyna Chem Kinet. 2021;7(2): 1–8.
- Liu, F, Teng, S, Song, R, Wang, S. Adsorption of methylene blue on anaerobic granular sludge: effect of functional groups. Desalination. 2010;263(1-3), 11-17
Figures:
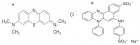
Figure 1

Figure 2
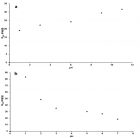
Figure 3
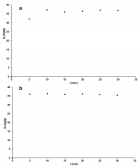
Figure 4
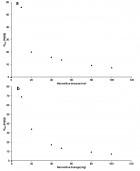
Figure 5
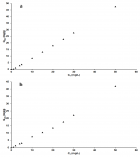
Figure 6

Figure 7

Figure 8

Figure 9

Figure 10
Similar Articles
-
Removal of Chromium from Aqueous Solution by Thermally Treated Mgal Layered Double HydroxideDashkhuu Khasbaatar*,Enkhtur Otgonjargal,Byambasuren Nyamsuren,Enkhtuul Surenjav,Gunchin Burmaa,Jadambaa Temuujin. Removal of Chromium from Aqueous Solution by Thermally Treated Mgal Layered Double Hydroxide. . 2017 doi: 10.29328/journal.acee.1001001; 1: 001-008
-
Behavior evaluation of freundlich and langmuir isotherms in cadmium preconcentration using solid phase extraction method for linear and nonlinear numerical computational patternsBenyamin Chahkandi,Mohammad Gheibi*,Amir Takhtravan. Behavior evaluation of freundlich and langmuir isotherms in cadmium preconcentration using solid phase extraction method for linear and nonlinear numerical computational patterns. . 2021 doi: 10.29328/journal.acee.1001029; 5: 007-010
-
Nano-silica from kaolinitic clay used as adsorbent for anionic and cationic dyes removal: linear and non-linear regression isotherms and kinetics studiesGustave Tchanang,Chantale Njiomou Djangang*,Charles Fon Abi,Danie Laure Mbella Moukouri,Guillonnel Trésor Nyadjou Djabo,Jean Marie Kepdieu,Philippe Blanchart. Nano-silica from kaolinitic clay used as adsorbent for anionic and cationic dyes removal: linear and non-linear regression isotherms and kinetics studies. . 2022 doi: 10.29328/journal.acee.1001034; 6: 008-018
-
Impact of dyes used in the mat on groundwater in and around Pattamadai, Tirunelveli district, TamilnaduGopikumar S*,Sundararajan S,Allwyn Kingsly Gladston J,Antony Vasantha Kumar C,Hari Babu K,Jeyakumar K. Impact of dyes used in the mat on groundwater in and around Pattamadai, Tirunelveli district, Tamilnadu. . 2023 doi: 10.29328/journal.acee.1001047; 7: 001-003
-
Isolation and Influence of Carbon Source on the Production of Extracellular Polymeric Substance by Bacteria for the Bioremediation of Heavy Metals in Santo Amaro CityLeila Thaise Santana de Oliveira Santos*, Kayque Frota Sampaio, Elisa Esposito, Elinalva Maciel Paulo, Aristóteles Góes-Neto, Amanda da Silva Souza, Taise Bomfim de Jesus. Isolation and Influence of Carbon Source on the Production of Extracellular Polymeric Substance by Bacteria for the Bioremediation of Heavy Metals in Santo Amaro City. . 2024 doi: 10.29328/journal.acee.1001060; 8: 012-017
Recently Viewed
-
Investigation of Fuel Cells under Transient (Dynamic) Conditions to Improve the Efficiency of Polymer Electrolyte Fuel Cells in Dead-Ended Anode Mode: Review ArticleParsa Rahimi*. Investigation of Fuel Cells under Transient (Dynamic) Conditions to Improve the Efficiency of Polymer Electrolyte Fuel Cells in Dead-Ended Anode Mode: Review Article. Ann Civil Environ Eng. 2025: doi: 10.29328/journal.acee.1001075; 9: 012-017
-
The Influence of Gravity on the Frequency of Processes in Various Geospheres of the Earth. Biogenic and Abiogenic Pathways of Formation of HC AccumulationsAA Ivlev*. The Influence of Gravity on the Frequency of Processes in Various Geospheres of the Earth. Biogenic and Abiogenic Pathways of Formation of HC Accumulations. Ann Civil Environ Eng. 2024: doi: 10.29328/journal.acee.1001067; 8: 052-056
-
Flood Risk Management in South-west Nigeria: Lagos as a Case StudyIdowu Michael*. Flood Risk Management in South-west Nigeria: Lagos as a Case Study. Ann Civil Environ Eng. 2024: doi: 10.29328/journal.acee.1001073; 8: 096-097
-
Post-stroke dizziness of visual-vestibular cortices originNobuhiro Inoue*,Satoshi Goto. Post-stroke dizziness of visual-vestibular cortices origin. J Neurosci Neurol Disord. 2020: doi: 10.29328/journal.jnnd.1001038; 4: 075-078
-
Complications of External Otitis in HorsesSchusser GF*, Kuhlmann CHR, Scheidemann W. Complications of External Otitis in Horses. Insights Vet Sci. 2024: doi: 10.29328/journal.ivs.1001041; 8: 012-014
Most Viewed
-
Feasibility study of magnetic sensing for detecting single-neuron action potentialsDenis Tonini,Kai Wu,Renata Saha,Jian-Ping Wang*. Feasibility study of magnetic sensing for detecting single-neuron action potentials. Ann Biomed Sci Eng. 2022 doi: 10.29328/journal.abse.1001018; 6: 019-029
-
Evaluation of In vitro and Ex vivo Models for Studying the Effectiveness of Vaginal Drug Systems in Controlling Microbe Infections: A Systematic ReviewMohammad Hossein Karami*, Majid Abdouss*, Mandana Karami. Evaluation of In vitro and Ex vivo Models for Studying the Effectiveness of Vaginal Drug Systems in Controlling Microbe Infections: A Systematic Review. Clin J Obstet Gynecol. 2023 doi: 10.29328/journal.cjog.1001151; 6: 201-215
-
Prospective Coronavirus Liver Effects: Available KnowledgeAvishek Mandal*. Prospective Coronavirus Liver Effects: Available Knowledge. Ann Clin Gastroenterol Hepatol. 2023 doi: 10.29328/journal.acgh.1001039; 7: 001-010
-
Causal Link between Human Blood Metabolites and Asthma: An Investigation Using Mendelian RandomizationYong-Qing Zhu, Xiao-Yan Meng, Jing-Hua Yang*. Causal Link between Human Blood Metabolites and Asthma: An Investigation Using Mendelian Randomization. Arch Asthma Allergy Immunol. 2023 doi: 10.29328/journal.aaai.1001032; 7: 012-022
-
An algorithm to safely manage oral food challenge in an office-based setting for children with multiple food allergiesNathalie Cottel,Aïcha Dieme,Véronique Orcel,Yannick Chantran,Mélisande Bourgoin-Heck,Jocelyne Just. An algorithm to safely manage oral food challenge in an office-based setting for children with multiple food allergies. Arch Asthma Allergy Immunol. 2021 doi: 10.29328/journal.aaai.1001027; 5: 030-037

HSPI: We're glad you're here. Please click "create a new Query" if you are a new visitor to our website and need further information from us.
If you are already a member of our network and need to keep track of any developments regarding a question you have already submitted, click "take me to my Query."